Mitochondrial respiratory chain disease discrimination by retrospective cohort analysis of blood metabolites
- PMID: 23920046
- PMCID: PMC3812452
- DOI: 10.1016/j.ymgme.2013.07.011
Mitochondrial respiratory chain disease discrimination by retrospective cohort analysis of blood metabolites
Abstract
Diagnosing primary mitochondrial respiratory chain (RC) dysfunction has long relied on invasive tissue biopsies, since no blood-based biomarker has been shown to have sufficiently high sensitivity and specificity across the myriad of individual clinical presentations. We sought to determine whether cohort-level evaluation of commonly obtained blood analytes might reveal consistent patterns to discriminate a heterogenous group of primary mitochondrial RC disease subjects both from control individuals and from subjects with pyruvate dehydrogenase deficiency.
Methods: Following IRB approval, 62 biochemical analyte concentrations or ratios were retrospectively analyzed in three well-defined and intentionally heterogeneous subject cohorts reflective of clinical practice: [1] Primary mitochondrial disease (n=19); [2] pyruvate dehydrogenase deficiency (n=4); and [3] controls (n=27). Blood analyte categories included comprehensive chemistry profile, creatine kinase, lipoprotein profile, lactate, pyruvate, and plasma amino acid profile. Non-parametric analyses were used to compare the median of each analyte level between cohorts.
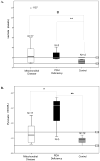
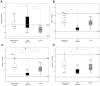
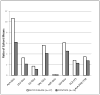
Results: Disease cohorts differed significantly in their median levels of triglycerides, lactate, pyruvate, and multiple individual plasma amino acids. Primary mitochondrial disease was significantly discriminated at the cohort level from pyruvate dehydrogenase deficiency by greater pyruvate and alanine elevation in pyruvate dehydrogenase deficiency, as well as significantly increased branched chain amino acid (BCAA) levels and increased ratios of individual BCAAs to glutamate in mitochondrial disease. In addition, significant elevation of median blood triglyceride level was seen in the primary mitochondrial disease cohort.
Conclusions: Blood metabolite profile analysis can discriminate a heterogeneous cohort of primary mitochondrial disease both from controls and from pyruvate dehydrogenase deficiency. Elevated BCAA levels, either absolutely or when considered relative to the level of glutamate, are common metabolic sequelae of primary mitochondrial RC disease. Prospective study is needed to validate observed plasma metabolite alterations as a potential biomarker of disease both in larger cohorts and at the individual subject level.
Keywords: BCAAs; Branched chain amino acids; CK; Metabolites; PC; PDH; PDHc; Primary human mitochondrial disease; Pyruvate dehydrogenase deficiency; RC; branched chain amino acids; creatine kinase; pyruvate carboxylase; pyruvate dehydrogenase; pyruvate dehydrogenase complex; respiratory chain.
© 2013.
Conflict of interest statement
Figures

References
-
- Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, Cohen BH. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. - PubMed
-
- Mordekar SR, Guthrie P, Bonham JR, Olpin SE, Hargreaves I, Baxter PS. The significance of reduced respiratory chain enzyme activities: clinical, biochemical and radiological associations. Eur J Paediatr Neurol. 2006;10:78–82. - PubMed
-
- Falk MJ. In: MITO 101 – Genetic Counseling for Mitochondrial Disease. Parikh S, DiMauro S, editors. United Mitochondrial Disease Foundation; 2008.
-
- Rotig A. Genetic bases of mitochondrial respiratory chain disorders. Diab Metab. 2010;36:97–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

