Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages
- PMID: 23921144
- PMCID: PMC3798699
- DOI: 10.1152/ajpendo.00251.2013
Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages
Abstract
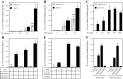
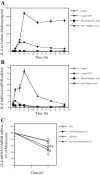
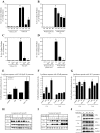
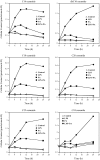
Periodontal disease is more prevalent and severe in patients with diabetes than in nondiabetic patients. In addition to diabetes, a large number of studies have demonstrated an association between obesity and chronic periodontal disease. However, the underlying mechanisms have not been well understood. Since plasma free fatty acids (FAs) are elevated in obese patients and saturated FAs such as palmitic acid (PA) have been shown to increase host inflammatory response, we sought to find out how PA interacts with lipopolysaccharide (LPS), an important pathological factor involved in periodontal disease, to enhance inflammation. We found that whereas low concentration of LPS (1 ng/ml) stimulated interleukin (IL)-6 expression in RAW 264.7 macrophages, PA further augmented it fourfold. Besides IL-6, PA amplified the stimulatory effect of LPS on a large amount of Toll-like receptor (TLR)4-mediated expression of proinflammatory signaling molecules such as IL-1 receptor-associated kinase-like 2 and proinflammatory molecules, including monocyte chemotactic protein-1 and colony-stimulating factor. We also observed that PA augmented TLR4 but not TLR2 signal, and the augmentation was mediated by nuclear factor-κB (NF-κB) pathways. To further elucidate the regulatory mechanism whereby PA amplifies LPS signal, our studies showed that PA and LPS synergistically increased hydrolysis of sphingomyelin by stimulating acid sphingomyelinase (ASMase) activity, which contributed to a marked increase in ceramide production and IL-6 upregulation. Taken together, this study has demonstrated that PA markedly augments TLR4-mediated proinflammatory signaling triggered by low concentration of LPS in macrophages, and ASMase plays a key role in the augmentation.
Keywords: fatty acid; inflammation; lipopolysaccharide; sphingomyelinase; toll-like receptor.
Figures

Similar articles
-
GPR40/FFA1 and neutral sphingomyelinase are involved in palmitate-boosted inflammatory response of microvascular endothelial cells to LPS.Atherosclerosis. 2015 May;240(1):163-73. doi: 10.1016/j.atherosclerosis.2015.03.013. Epub 2015 Mar 14. Atherosclerosis. 2015. PMID: 25795558 Free PMC article.
-
Lipopolysaccharide and palmitic acid synergistically induced MCP-1 production via MAPK-meditated TLR4 signaling pathway in RAW264.7 cells.Lipids Health Dis. 2019 Mar 25;18(1):71. doi: 10.1186/s12944-019-1017-4. Lipids Health Dis. 2019. PMID: 30909920 Free PMC article.
-
Anti-Inflammatory Effect of Auranofin on Palmitic Acid and LPS-Induced Inflammatory Response by Modulating TLR4 and NOX4-Mediated NF-κB Signaling Pathway in RAW264.7 Macrophages.Int J Mol Sci. 2021 May 31;22(11):5920. doi: 10.3390/ijms22115920. Int J Mol Sci. 2021. PMID: 34072916 Free PMC article.
-
The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation.Mediators Inflamm. 2016;2016:6978936. doi: 10.1155/2016/6978936. Epub 2016 May 18. Mediators Inflamm. 2016. PMID: 27293318 Free PMC article. Review.
-
Acid sphingomyelinase in macrophage biology.Cell Mol Life Sci. 2011 Oct;68(20):3293-305. doi: 10.1007/s00018-011-0686-6. Epub 2011 May 2. Cell Mol Life Sci. 2011. PMID: 21533981 Free PMC article. Review.
Cited by
-
siRNA-Mediated Timp1 Silencing Inhibited the Inflammatory Phenotype during Acute Lung Injury.Int J Mol Sci. 2023 Jan 13;24(2):1641. doi: 10.3390/ijms24021641. Int J Mol Sci. 2023. PMID: 36675165 Free PMC article.
-
Amitriptyline inhibits nonalcoholic steatohepatitis and atherosclerosis induced by high-fat diet and LPS through modulation of sphingolipid metabolism.Am J Physiol Endocrinol Metab. 2020 Feb 1;318(2):E131-E144. doi: 10.1152/ajpendo.00181.2019. Epub 2019 Dec 10. Am J Physiol Endocrinol Metab. 2020. PMID: 31821039 Free PMC article.
-
Inhibition of acid sphingomyelinase by imipramine abolishes the synergy between metabolic syndrome and periodontitis on alveolar bone loss.J Periodontal Res. 2022 Jan;57(1):173-185. doi: 10.1111/jre.12951. Epub 2021 Nov 8. J Periodontal Res. 2022. PMID: 34748647 Free PMC article.
-
Simvastatin inhibits LPS-induced alveolar bone loss during metabolic syndrome.J Dent Res. 2014 Mar;93(3):294-9. doi: 10.1177/0022034513516980. Epub 2013 Dec 18. J Dent Res. 2014. PMID: 24352501 Free PMC article.
-
A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes.Am J Clin Nutr. 2018 Jun 1;107(6):1017-1026. doi: 10.1093/ajcn/nqy051. Am J Clin Nutr. 2018. PMID: 29868913 Free PMC article.
References
-
- Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev 127: 25–50, 1992 - PubMed
-
- Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol 270: 109–120, 2002 - PubMed
-
- Bullon P, Morillo JM, Ramirez-Tortosa MC, Quiles JL, Newman HN, Battino M. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res 88: 503–518, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

