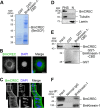
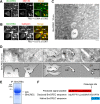
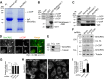
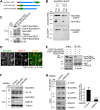
BmCREC is an endoplasmic reticulum (ER) resident protein and required for ER/Golgi morphology
- PMID: 23921381
- PMCID: PMC3772211
- DOI: 10.1074/jbc.M113.463018
BmCREC is an endoplasmic reticulum (ER) resident protein and required for ER/Golgi morphology
Abstract
Silkworm posterior silkgland is a model for studying intracellular trafficking. Here, using this model, we identify several potential cargo proteins of BmKinesin-1 and focus on one candidate, BmCREC. BmCREC (also known as Bombyx mori DNA supercoiling factor, BmSCF) was previously proposed to supercoil DNA in the nucleus. However, we show here that BmCREC is localized in the ER lumen. Its C-terminal tetrapeptide HDEF is recognized by the KDEL receptor, and subsequently it is retrogradely transported by coat protein I (COPI) vesicles to the ER. Lacking the HDEF tetrapeptide of BmCREC or knocking down COPI subunits results in decreased ER retention and simultaneously increased secretion of BmCREC. Furthermore, we find that BmCREC knockdown markedly disrupts the morphology of the ER and Golgi apparatus and leads to a defect of posterior silkgland tube expansion. Together, our results clarify the ER retention mechanism of BmCREC and reveal that BmCREC is indispensable for maintaining ER/Golgi morphology.
Keywords: BmCREC; COPI; Endoplasmic Reticulum (ER); Golgi; Intracellular Trafficking; KDEL Receptor; Kinesin; PSG Tube Expansion; Vesicles.
Figures

Similar articles
-
Silkworm coatomers and their role in tube expansion of posterior silkgland.PLoS One. 2010 Oct 12;5(10):e13252. doi: 10.1371/journal.pone.0013252. PLoS One. 2010. PMID: 20967265 Free PMC article.
-
COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGFR nuclear transport.Biochem Biophys Res Commun. 2010 Sep 3;399(4):498-504. doi: 10.1016/j.bbrc.2010.07.096. Epub 2010 Jul 30. Biochem Biophys Res Commun. 2010. PMID: 20674546 Free PMC article.
-
A role for kinesin-2 in COPI-dependent recycling between the ER and the Golgi complex.Curr Biol. 2006 Nov 21;16(22):2245-51. doi: 10.1016/j.cub.2006.09.060. Curr Biol. 2006. PMID: 17113389
-
Breaking the COPI monopoly on Golgi recycling.Trends Cell Biol. 2000 Sep;10(9):385-91. doi: 10.1016/s0962-8924(00)01818-3. Trends Cell Biol. 2000. PMID: 10932096 Review.
-
ER-to-Golgi transport and cytoskeletal interactions in animal cells.Cell Mol Life Sci. 2004 Jan;61(2):133-45. doi: 10.1007/s00018-003-3352-9. Cell Mol Life Sci. 2004. PMID: 14745493 Free PMC article. Review.
References
-
- Bonifacino J. S., Lippincott-Schwartz J. (2003) Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 4, 409–414 - PubMed
-
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R. (2004) Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20, 87–123 - PubMed
-
- Styers M. L., O'Connor A. K., Grabski R., Cormet-Boyaka E., Sztul E. (2008) Depletion of β-COP reveals a role for COP-I in compartmentalization of secretory compartments and in biosynthetic transport of caveolin-1. Am. J. Physiol. Cell Physiol. 294, C1485–1498 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

