ATP and AMP mutually influence their interaction with the ATP-binding cassette (ABC) adenylate kinase cystic fibrosis transmembrane conductance regulator (CFTR) at separate binding sites
- PMID: 23921386
- PMCID: PMC3779764
- DOI: 10.1074/jbc.M113.479675
ATP and AMP mutually influence their interaction with the ATP-binding cassette (ABC) adenylate kinase cystic fibrosis transmembrane conductance regulator (CFTR) at separate binding sites
Abstract
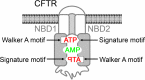
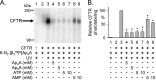
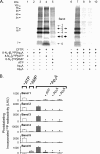
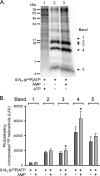
Cystic fibrosis transmembrane conductance regulator (CFTR) is an anion channel in the ATP-binding cassette (ABC) transporter protein family. In the presence of ATP and physiologically relevant concentrations of AMP, CFTR exhibits adenylate kinase activity (ATP + AMP &lrarr2; 2 ADP). Previous studies suggested that the interaction of nucleotide triphosphate with CFTR at ATP-binding site 2 is required for this activity. Two other ABC proteins, Rad50 and a structural maintenance of chromosome protein, also have adenylate kinase activity. All three ABC adenylate kinases bind and hydrolyze ATP in the absence of other nucleotides. However, little is known about how an ABC adenylate kinase interacts with ATP and AMP when both are present. Based on data from non-ABC adenylate kinases, we hypothesized that ATP and AMP mutually influence their interaction with CFTR at separate binding sites. We further hypothesized that only one of the two CFTR ATP-binding sites is involved in the adenylate kinase reaction. We found that 8-azidoadenosine 5'-triphosphate (8-N3-ATP) and 8-azidoadenosine 5'-monophosphate (8-N3-AMP) photolabeled separate sites in CFTR. Labeling of the AMP-binding site with 8-N3-AMP required the presence of ATP. Conversely, AMP enhanced photolabeling with 8-N3-ATP at ATP-binding site 2. The adenylate kinase active center probe P(1),P(5)-di(adenosine-5') pentaphosphate interacted simultaneously with an AMP-binding site and ATP-binding site 2. These results show that ATP and AMP interact with separate binding sites but mutually influence their interaction with the ABC adenylate kinase CFTR. They further indicate that the active center of the adenylate kinase comprises ATP-binding site 2.
Keywords: ABC Transporter; AMP; ATP; CFTR; Cystic fibrosis; Genetic Diseases.
Figures


Similar articles
-
Mutating the Conserved Q-loop Glutamine 1291 Selectively Disrupts Adenylate Kinase-dependent Channel Gating of the ATP-binding Cassette (ABC) Adenylate Kinase Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and Reduces Channel Function in Primary Human Airway Epithelia.J Biol Chem. 2015 May 29;290(22):14140-53. doi: 10.1074/jbc.M114.611616. Epub 2015 Apr 17. J Biol Chem. 2015. PMID: 25887396 Free PMC article.
-
Demonstration of phosphoryl group transfer indicates that the ATP-binding cassette (ABC) transporter cystic fibrosis transmembrane conductance regulator (CFTR) exhibits adenylate kinase activity.J Biol Chem. 2012 Oct 19;287(43):36105-10. doi: 10.1074/jbc.M112.408450. Epub 2012 Sep 4. J Biol Chem. 2012. PMID: 22948143 Free PMC article.
-
An intrinsic adenylate kinase activity regulates gating of the ABC transporter CFTR.Cell. 2003 Dec 26;115(7):837-50. doi: 10.1016/s0092-8674(03)00983-8. Cell. 2003. PMID: 14697202
-
Role of CFTR's intrinsic adenylate kinase activity in gating of the Cl(-) channel.J Bioenerg Biomembr. 2007 Dec;39(5-6):473-9. doi: 10.1007/s10863-007-9119-5. J Bioenerg Biomembr. 2007. PMID: 17965924 Review.
-
Exploiting species differences to understand the CFTR Cl- channel.Biochem Soc Trans. 2015 Oct;43(5):975-82. doi: 10.1042/BST20150129. Biochem Soc Trans. 2015. PMID: 26517912 Review.
Cited by
-
Coupled ATPase-adenylate kinase activity in ABC transporters.Nat Commun. 2016 Dec 22;7:13864. doi: 10.1038/ncomms13864. Nat Commun. 2016. PMID: 28004795 Free PMC article.
-
NM23 proteins: innocent bystanders or local energy boosters for CFTR?Lab Invest. 2018 Mar;98(3):272-282. doi: 10.1038/labinvest.2017.121. Epub 2017 Dec 18. Lab Invest. 2018. PMID: 29251738 Review.
-
The Alternating Access Mechanism in Mammalian Multidrug Resistance Transporters and Their Bacterial Homologs.Membranes (Basel). 2023 May 30;13(6):568. doi: 10.3390/membranes13060568. Membranes (Basel). 2023. PMID: 37367772 Free PMC article.
-
A cluster of inhibitory residues in the regulatory domain prevents activation of the cystic fibrosis transmembrane conductance regulator.J Biol Chem. 2025 May;301(5):108460. doi: 10.1016/j.jbc.2025.108460. Epub 2025 Mar 26. J Biol Chem. 2025. PMID: 40154618 Free PMC article.
-
Mutating the Conserved Q-loop Glutamine 1291 Selectively Disrupts Adenylate Kinase-dependent Channel Gating of the ATP-binding Cassette (ABC) Adenylate Kinase Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and Reduces Channel Function in Primary Human Airway Epithelia.J Biol Chem. 2015 May 29;290(22):14140-53. doi: 10.1074/jbc.M114.611616. Epub 2015 Apr 17. J Biol Chem. 2015. PMID: 25887396 Free PMC article.
References
-
- Hanrahan J. W., Gentzsch M., Riordan J. R. (2003) The cystic fibrosis transmembrane conductance regulator (ABCC7). in ABC Proteins from Bacteria to Man (Holland I. B., Cole S. P. C., Kuchler K., Higgins C. F., eds) pp. 589–618, Academic Press, Amsterdam
-
- Shyamala V., Baichwal V., Beall E., Ames G. F. (1991) Structure-function analysis of the histidine permease and comparison with cystic fibrosis mutations. J. Biol. Chem. 266, 18714–18719 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

