The ENTPD5/mt-PCPH oncoprotein is a catalytically inactive member of the ectonucleoside triphosphate diphosphohydrolase family
- PMID: 23921441
- PMCID: PMC3829800
- DOI: 10.3892/ijo.2013.2052
The ENTPD5/mt-PCPH oncoprotein is a catalytically inactive member of the ectonucleoside triphosphate diphosphohydrolase family
Abstract
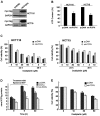
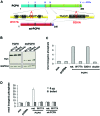
Expression of the ENTPD5/mt-PCPH onco-protein and overexpression of the normal ENTPD5/PCPH protein contribute to the malignant transformation of diverse mammalian cell types, and PCPH is mutated and/or deregulated in various human tumor types. Expression of PCPH or mt-PCPH caused similar phenotypes, yet the effects promoted by mt-PCPH expression were consistently and substantially greater. ATP depletion and increased stress‑resistance are phenotypes commonly associated with PCPH and mt-PCPH expression. It was suggested that the intrinsic nucleoside triphosphate diphosphohydrolase (NTPDase) activity of PCPH and mt-PCPH may be responsible for these phenotypes, but direct supporting evidence remains to be established. Results from experiments designed to test such hypothesis demonstrate that, as expected, mt-PCPH expression in human colorectal carcinoma (CRC) cells decreased their ATP levels and conferred resistance to oxaliplatin, a colorectal cancer-relevant chemotherapeutic agent. Using a combination of site-directed mutagenesis, immunoprecipitation methods, in vitro enzyme activity assays and in situ enzyme activity determinations in live cells, this report also demonstrates that the mt-PCPH oncoprotein lacks detectable NTPDase activity, indicating that direct ATP cleavage by mt-PCPH did not cause the ATP depletion observed in mt-PCPH-expressing CRC cells. These results strongly suggest that the mt-PCPH oncoprotein may regulate the cellular energy levels and subsequent chemoresistance by an NTPDase-independent mechanism. Understanding possible alternative mechanisms will be essential to devise strategies for the successful treatment of predictably therapeutically resistant tumors expressing either increased PCPH levels or, particularly, the mt-PCPH oncoprotein.
Figures



Similar articles
-
NTPDase5/PCPH as a new target in highly aggressive tumors: a systematic review.Biomed Res Int. 2014;2014:123010. doi: 10.1155/2014/123010. Epub 2014 Jun 23. Biomed Res Int. 2014. PMID: 25045656 Free PMC article.
-
Identity between the PCPH proto-oncogene and the CD39L4 (ENTPD5) ectonucleoside triphosphate diphosphohydrolase gene.Int J Oncol. 2001 Dec;19(6):1249-54. doi: 10.3892/ijo.19.6.1249. Int J Oncol. 2001. PMID: 11713596
-
Both normal and transforming PCPH proteins have guanosine diphosphatase activity but only the oncoprotein cooperates with Ras in activating extracellular signal-regulated kinase ERK1.Cancer Res. 2000 Mar 15;60(6):1720-8. Cancer Res. 2000. PMID: 10749145
-
PCPH/ENTPD5 expression confers to prostate cancer cells resistance against cisplatin-induced apoptosis through protein kinase Calpha-mediated Bcl-2 stabilization.Cancer Res. 2009 Jan 1;69(1):102-10. doi: 10.1158/0008-5472.CAN-08-2922. Cancer Res. 2009. PMID: 19117992 Free PMC article.
-
[Ecto-nucleotidases of ectonucleoside triphosphate diphosphohydrolase family: structure, localization and functional significance].Ukr Biokhim Zh (1999). 2010 May-Jun;82(3):5-17. Ukr Biokhim Zh (1999). 2010. PMID: 21328873 Review. Ukrainian.
Cited by
-
miRNA Expression Signatures of Therapy Response in Squamous Cell Carcinomas.Cancers (Basel). 2020 Dec 28;13(1):63. doi: 10.3390/cancers13010063. Cancers (Basel). 2020. PMID: 33379285 Free PMC article.
-
NTPDase5/PCPH as a new target in highly aggressive tumors: a systematic review.Biomed Res Int. 2014;2014:123010. doi: 10.1155/2014/123010. Epub 2014 Jun 23. Biomed Res Int. 2014. PMID: 25045656 Free PMC article.
-
Predicting human disease mutations and identifying drug targets from mouse gene knockout phenotyping campaigns.Dis Model Mech. 2019 May 7;12(5):dmm038224. doi: 10.1242/dmm.038224. Dis Model Mech. 2019. PMID: 31064765 Free PMC article. Review.
-
[ENTPD5 gene is highly expressed in epithelial ovarian cancer: analysis based on Oncomine database and bioinformatics].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Apr 20;41(4):555-561. doi: 10.12122/j.issn.1673-4254.2021.04.11. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 33963715 Free PMC article. Chinese.
-
ENTPD5 induces apoptosis in lung cancer cells via regulating caspase 3 expression.PLoS One. 2015 Mar 20;10(3):e0120046. doi: 10.1371/journal.pone.0120046. eCollection 2015. PLoS One. 2015. PMID: 25794010 Free PMC article.
References
-
- Cairns RA , Harris IS , Mak TW . Regulation of cancer cell metabolism . Nat Rev Cancer . 2011 ; 11 : 85 – 95 . - PubMed
-
- Warburg O . On respiratory impairment in cancer cells . Science . 1956 ; 124 : 269 – 270 . - PubMed
-
- Sattler UG , Hirschhaeuser F , Mueller-Klieser WF . Manipulation of glycolysis in malignant tumors: fantasy or therapy? . Curr Med Chem . 2010 ; 17 : 96 – 108 . - PubMed
-
- Chan EC , Koh PK , Mal M , et al. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS) . J Proteome Res . 2009 ; 8 : 352 – 361 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

