A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines
- PMID: 23921513
- PMCID: PMC3906413
- DOI: 10.4161/hv.25181
A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines
Abstract
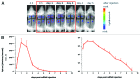
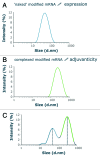
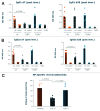
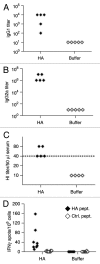
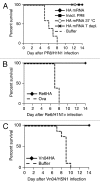
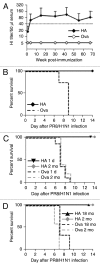
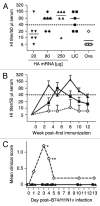
Nucleotide based vaccines represent an enticing, novel approach to vaccination. We have developed a novel immunization technology, RNActive(®) vaccines, that have two important characteristics: mRNA molecules are used whose protein expression capacity has been enhanced by 4 to 5 orders of magnitude by modifications of the nucleotide sequence with the naturally occurring nucleotides A (adenosine), G (guanosine), C (cytosine), U (uridine) that do not affect the primary amino acid sequence. Second, they are complexed with protamine and thus activate the immune system by involvement of toll-like receptor (TLR) 7. Essentially, this bestows self-adjuvant activity on RNActive(®) vaccines. RNActive(®) vaccines induce strong, balanced immune responses comprising humoral and cellular responses, effector and memory responses as well as activation of important subpopulations of immune cells, such as Th1 and Th2 cells. Pre-germinal center and germinal center B cells were detected in human patients upon vaccination. RNActive(®) vaccines successfully protect against lethal challenges with a variety of different influenza strains in preclinical models. Anti-tumor activity was observed preclinically under therapeutic as well as prophylactic conditions. Initial clinical experiences suggest that the preclinical immunogenicity of RNActive(®) could be successfully translated to humans.
Keywords: RNA; vaccines.
Figures



References
-
- Conry RM, LoBuglio AF, Wright M, Sumerel L, Pike MJ, Johanning F, et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55:1397–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
