A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells
- PMID: 23921636
- PMCID: PMC3799453
- DOI: 10.1093/nar/gkt706
A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells
Abstract
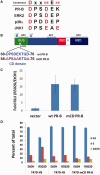
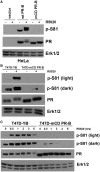
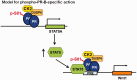
Progesterone receptors (PR) are transcription factors relevant to breast cancer biology. Herein, we describe an N-terminal common docking (CD) domain in PR-B, a motif first described in mitogen-activated protein kinases. Binding studies revealed PR-B interacts with dual-specificity phosphatase 6 (DUSP6) via the CD domain. Mutation of the PR-B CD domain (mCD) attenuated cell cycle progression and expression of PR-B target genes (including STAT5A and Wnt1); mCD PR-B failed to undergo phosphorylation on Ser81, a ck2-dependent site required for expression of these genes. PR-B Ser81 phosphorylation was dependent on binding with DUSP6 and required for recruitment of a transcriptional complex consisting of PR-B, DUSP6 and ck2 to an enhancer region upstream of the Wnt1 promoter. STAT5 was present at this site in the absence or presence of progestin. Furthermore, phospho-Ser81 PR-B was recruited to the STAT5A gene upon progestin treatment, suggestive of a feed-forward mechanism. Inhibition of JAK/STAT-signaling blocked progestin-induced STAT5A and Wnt1 expression. Our studies show that DUSP6 serves as a scaffold for ck2-dependent PR-B Ser81 phosphorylation and subsequent PR-B-specific gene selection in coordination with STAT5. Coregulation of select target genes by PR-B and STAT5 is likely a global mechanism required for growth promoting programs relevant to mammary stem cell biology and cancer.
Figures





Similar articles
-
ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells.Mol Cell Biol. 2011 Jun;31(12):2439-52. doi: 10.1128/MCB.01246-10. Epub 2011 Apr 25. Mol Cell Biol. 2011. PMID: 21518957 Free PMC article.
-
Progesterone induction of the 11beta-hydroxysteroid dehydrogenase type 2 promoter in breast cancer cells involves coordinated recruitment of STAT5A and progesterone receptor to a distal enhancer and polymerase tracking.Mol Cell Biol. 2008 Jun;28(11):3830-49. doi: 10.1128/MCB.01217-07. Epub 2008 Mar 31. Mol Cell Biol. 2008. PMID: 18378698 Free PMC article.
-
Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors.Mol Endocrinol. 2008 Apr;22(4):823-37. doi: 10.1210/me.2007-0437. Epub 2008 Jan 17. Mol Endocrinol. 2008. PMID: 18202149 Free PMC article.
-
Signaling inputs to progesterone receptor gene regulation and promoter selectivity.Mol Cell Endocrinol. 2009 Sep 24;308(1-2):47-52. doi: 10.1016/j.mce.2009.01.004. Epub 2009 Jan 20. Mol Cell Endocrinol. 2009. PMID: 19549591 Free PMC article. Review.
-
Chromatin remodeling and control of cell proliferation by progestins via cross talk of progesterone receptor with the estrogen receptors and kinase signaling pathways.Ann N Y Acad Sci. 2006 Nov;1089:59-72. doi: 10.1196/annals.1386.025. Ann N Y Acad Sci. 2006. PMID: 17261755 Review.
Cited by
-
Rapid estrogen signaling negatively regulates PTEN activity through phosphorylation in endometrial cancer cells.Horm Cancer. 2014 Aug;5(4):218-31. doi: 10.1007/s12672-014-0184-z. Epub 2014 May 21. Horm Cancer. 2014. PMID: 24844349 Free PMC article.
-
Ulipristal Acetate Inhibits Progesterone Receptor Isoform A-Mediated Human Breast Cancer Proliferation and BCl2-L1 Expression.PLoS One. 2015 Oct 16;10(10):e0140795. doi: 10.1371/journal.pone.0140795. eCollection 2015. PLoS One. 2015. PMID: 26474308 Free PMC article.
-
Progesterone receptor isoform-dependent cross-talk between prolactin and fatty acid synthase in breast cancer.Aging (Albany NY). 2020 Dec 10;12(24):24671-24692. doi: 10.18632/aging.202289. Epub 2020 Dec 10. Aging (Albany NY). 2020. PMID: 33335078 Free PMC article.
-
Heregulin Co-opts PR Transcriptional Action Via Stat3 Role As a Coregulator to Drive Cancer Growth.Mol Endocrinol. 2015 Oct;29(10):1468-85. doi: 10.1210/me.2015-1170. Epub 2015 Sep 4. Mol Endocrinol. 2015. PMID: 26340407 Free PMC article.
-
Steroid Receptors in Breast Cancer: Understanding of Molecular Function as a Basis for Effective Therapy Development.Cancers (Basel). 2021 Sep 24;13(19):4779. doi: 10.3390/cancers13194779. Cancers (Basel). 2021. PMID: 34638264 Free PMC article. Review.
References
-
- Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol. Endocrinol. 1993;7:1603–1616. - PubMed
-
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol. Endocrinol. 1994;8:1347–1360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

