Analog of somatostatin vapreotide exhibits biological effects in vitro via interaction with neurokinin-1 receptor
- PMID: 23921645
- PMCID: PMC3839635
- DOI: 10.1159/000350468
Analog of somatostatin vapreotide exhibits biological effects in vitro via interaction with neurokinin-1 receptor
Abstract
Objectives: Vapreotide, a synthetic analog of somatostatin, has analgesic activity most likely mediated through the blockade of neurokinin-1 receptor (NK1R), the substance P (SP)-preferring receptor. The ability of vapreotide to interfere with other biological effects of SP has yet to be investigated.
Methods: We studied the ability of vapreotide to antagonize NK1R in three different cell types: immortalized U373MG human astrocytoma cells, human monocyte-derived macrophages (MDM) and a human embryonic kidney cell line, HEK293. Both U373MG and MDM express endogenous NK1R while HEK293 cells, which normally do not express NK1R, are stably transformed to express human NK1R (HEK293-NK1R).
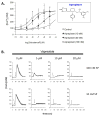
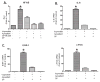
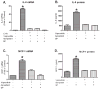
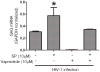
Results: Vapreotide attenuates SP-triggered intracellular calcium increases and nuclear factor-κB activation in a dose-dependent manner. Vapreotide also inhibits SP-induced interleukin-8 and monocyte chemotactic protein-1 production in HEK293-NK1R and U373MG cell lines. Vapreotide inhibits HIV-1 infection of human MDM in vitro, an effect that is reversible by SP pretreatment.
Conclusions: Our findings indicate that vapreotide has NK1R antagonist activity and may have a potential application as a therapeutic intervention in HIV-1 infection.
Copyright © 2013 S. Karger AG, Basel.
Figures
Similar articles
-
Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo.J Hepatol. 2014 May;60(5):985-94. doi: 10.1016/j.jhep.2013.12.024. Epub 2014 Jan 8. J Hepatol. 2014. PMID: 24412605
-
The SP/NK1R system promotes the proliferation of breast cancer cells through NF-κB-mediated inflammatory responses.Cell Biochem Biophys. 2023 Dec;81(4):787-794. doi: 10.1007/s12013-023-01171-y. Epub 2023 Sep 23. Cell Biochem Biophys. 2023. PMID: 37740877
-
Potential in vitro therapeutic effects of targeting SP/NK1R system in cervical cancer.Mol Biol Rep. 2022 Feb;49(2):1067-1076. doi: 10.1007/s11033-021-06928-3. Epub 2021 Nov 12. Mol Biol Rep. 2022. PMID: 34766230
-
Substance P and the Neurokinin-1 Receptor: The New CRF.Int Rev Neurobiol. 2017;136:151-175. doi: 10.1016/bs.irn.2017.06.008. Epub 2017 Aug 18. Int Rev Neurobiol. 2017. PMID: 29056150 Review.
-
The redox modulatory effects of SP/NK1R system: Implications for oxidative stress-associated disorders.Life Sci. 2022 May 1;296:120448. doi: 10.1016/j.lfs.2022.120448. Epub 2022 Mar 2. Life Sci. 2022. PMID: 35247438 Review.
Cited by
-
Reduction of soluble CD163, substance P, programmed death 1 and inflammatory markers: phase 1B trial of aprepitant in HIV-1-infected adults.AIDS. 2015 May 15;29(8):931-9. doi: 10.1097/QAD.0000000000000638. AIDS. 2015. PMID: 25915168 Free PMC article. Clinical Trial.
-
Agonists, Antagonists and Receptors of Somatostatin: Pathophysiological and Therapeutical Implications in Neoplasias.Curr Issues Mol Biol. 2024 Sep 2;46(9):9721-9759. doi: 10.3390/cimb46090578. Curr Issues Mol Biol. 2024. PMID: 39329930 Free PMC article. Review.
References
-
- Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. - PubMed
-
- Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992;41:24–30. - PubMed
-
- Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance p and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. - PubMed
-
- Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance p and its receptor. J Neuroimmunol. 1998;86:80–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

