Strategies for optimizing water-exchange rates of lanthanide-based contrast agents for magnetic resonance imaging
- PMID: 23921796
- PMCID: PMC3775326
- DOI: 10.3390/molecules18089352
Strategies for optimizing water-exchange rates of lanthanide-based contrast agents for magnetic resonance imaging
Abstract
This review describes recent advances in strategies for tuning the water-exchange rates of contrast agents for magnetic resonance imaging (MRI). Water-exchange rates play a critical role in determining the efficiency of contrast agents; consequently, optimization of water-exchange rates, among other parameters, is necessary to achieve high efficiencies. This need has resulted in extensive research efforts to modulate water-exchange rates by chemically altering the coordination environments of the metal complexes that function as contrast agents. The focus of this review is coordination-chemistry-based strategies used to tune the water-exchange rates of lanthanide(III)-based contrast agents for MRI. Emphasis will be given to results published in the 21st century, as well as implications of these strategies on the design of contrast agents.
Figures




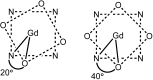
Similar articles
-
PARACEST agents: modulating MRI contrast via water proton exchange.Acc Chem Res. 2003 Oct;36(10):783-90. doi: 10.1021/ar020228m. Acc Chem Res. 2003. PMID: 14567712 Review.
-
The importance of water exchange rates in the design of responsive agents for MRI.Curr Opin Chem Biol. 2013 Apr;17(2):167-74. doi: 10.1016/j.cbpa.2012.12.012. Epub 2013 Jan 17. Curr Opin Chem Biol. 2013. PMID: 23333571 Free PMC article. Review.
-
Modulating water-exchange rates of lanthanide(III)-containing polyaminopolycarboxylate-type complexes using polyethylene glycol.Dalton Trans. 2013 May 21;42(19):6724-7. doi: 10.1039/c3dt50885d. Dalton Trans. 2013. PMID: 23584014 Free PMC article.
-
Cyclen lanthanide-based micellar structures for application as luminescent [Eu(iii)] and magnetic [Gd(iii)] resonance imaging (MRI) contrast agents.Chem Commun (Camb). 2016 Sep 18;52(72):10858-61. doi: 10.1039/c6cc03092k. Epub 2016 Aug 15. Chem Commun (Camb). 2016. PMID: 27523566
-
Isoquinoline-based lanthanide complexes: bright NIR optical probes and efficient MRI agents.Inorg Chem. 2012 Feb 20;51(4):2522-32. doi: 10.1021/ic202446e. Epub 2012 Jan 10. Inorg Chem. 2012. PMID: 22233349
Cited by
-
Chiral DOTA chelators as an improved platform for biomedical imaging and therapy applications.Nat Commun. 2018 Feb 27;9(1):857. doi: 10.1038/s41467-018-03315-8. Nat Commun. 2018. PMID: 29487362 Free PMC article.
-
A (Fluoroalkyl)Guanidine Modulates the Relaxivity of a Phosphonate-Containing T1-Shortening Contrast Agent.J Fluor Chem. 2014 Dec 1;168:177-183. doi: 10.1016/j.jfluchem.2014.09.018. J Fluor Chem. 2014. PMID: 25431503 Free PMC article.
-
Manganese-Enhanced MRI: Biological Applications in Neuroscience.Front Neurol. 2015 Jul 10;6:161. doi: 10.3389/fneur.2015.00161. eCollection 2015. Front Neurol. 2015. PMID: 26217304 Free PMC article. Review.
-
Design and synthesis of chiral DOTA-based MRI contrast agents with remarkable relaxivities.Commun Chem. 2023 Nov 16;6(1):251. doi: 10.1038/s42004-023-01050-w. Commun Chem. 2023. PMID: 37973896 Free PMC article.
-
Aqueous Lanthanide Chemistry in Asymmetric Catalysis and Magnetic Resonance Imaging.Synlett. 2016 Jun;27(9):1310-1317. doi: 10.1055/s-0035-1561363. Synlett. 2016. PMID: 27493448 Free PMC article.
References
-
- Hermann P., Kotek J., Kubíček V., Lukeš I. Gadolinium(III) complexes as MRI contrast agents: ligand design and properties of the complexes. Dalton Trans. 2008:3027–3047. - PubMed
-
- Laurent S., Henoumont C., Vander Elst L., Muller R.N. Synthesis and physicochemical characterization of Gd-DTPA derivatives as contrast agents for MRI. Eur. J. Inorg. Chem. 2012;2012:1889–1915.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

