The role of PARL and HtrA2 in striatal neuronal injury after transient global cerebral ischemia
- PMID: 23921894
- PMCID: PMC3824183
- DOI: 10.1038/jcbfm.2013.139
The role of PARL and HtrA2 in striatal neuronal injury after transient global cerebral ischemia
Abstract
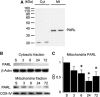
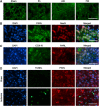
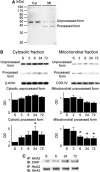
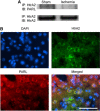
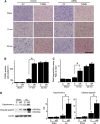
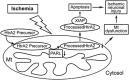
The presenilin-associated rhomboid-like (PARL) protein and high temperature requirement factor A2 (HtrA2) are key regulators of mitochondrial integrity and play pivotal roles in apoptosis. However, their roles after cerebral ischemia have not been thoroughly elucidated. To clarify these roles, mice were subjected to transient global cerebral ischemia, and striatal neuronal injury was assessed. Western blot and coimmunoprecipitation analyses revealed that PARL and processed HtrA2 localized to mitochondria, and that PARL was bound to HtrA2 in sham animals. Expression of PARL and processed HtrA2 in mitochondria significantly decreased 6 to 72 hours after ischemia, and the binding of PARL to HtrA2 disappeared after ischemia. In contrast, expression of processed HtrA2 increased 24 hours after ischemia in the cytosol, where HtrA2 was bound to X chromosome-linked inhibitor-of-apoptosis protein (XIAP). Administration of PARL small interfering RNA inhibited HtrA2 processing and worsened ischemic neuronal injury. Our results show that downregulation of PARL after ischemia is a key step in ischemic neuronal injury, and that it decreases HtrA2 processing and increases neuronal vulnerability. In addition, processed HtrA2 released into the cytosol after ischemia contributes to neuronal injury via inhibition of XIAP.
Figures

Comment in
-
PARL and HtrA2: another intricate ischemic neuronal apoptotic process starting within mitochondria.J Cereb Blood Flow Metab. 2013 Nov;33(11):1657. doi: 10.1038/jcbfm.2013.138. Epub 2013 Aug 7. J Cereb Blood Flow Metab. 2013. PMID: 23921904 Free PMC article. No abstract available.
References
-
- Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med. 2004;25:365–451. - PubMed
-
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. - PubMed
-
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. - PubMed
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

