Silencing PP2A inhibitor by lenti-shRNA interference ameliorates neuropathologies and memory deficits in tg2576 mice
- PMID: 23922015
- PMCID: PMC3863796
- DOI: 10.1038/mt.2013.189
Silencing PP2A inhibitor by lenti-shRNA interference ameliorates neuropathologies and memory deficits in tg2576 mice
Abstract
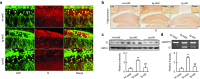
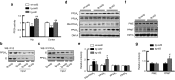
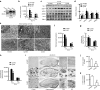
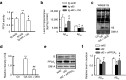
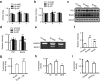
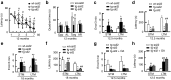
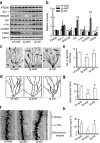
Deficits of protein phosphatase-2A (PP2A) play a crucial role in tau hyperphosphorylation, amyloid overproduction, and synaptic suppression of Alzheimer's disease (AD), in which PP2A is inactivated by the endogenously increased inhibitory protein, namely inhibitor-2 of PP2A (I2(PP2A)). Therefore, in vivo silencing I2(PP2A) may rescue PP2A and mitigate AD neurodegeneration. By infusion of lentivirus-shRNA targeting I2(PP2A) (LV-siI2(PP2A)) into hippocampus and frontal cortex of 11-month-old tg2576 mice, we demonstrated that expression of LV-siI2(PP2A) decreased remarkably the elevated I2(PP2A) in both mRNA and protein levels. Simultaneously, the PP2A activity was restored with the mechanisms involving reduction of the inhibitory binding of I2(PP2A) to PP2A catalytic subunit (PP2AC), repression of the inhibitory Leu309-demethylation and elevation of PP2AC. Silencing I2(PP2A) induced a long-lasting attenuation of amyloidogenesis in tg2576 mice with inhibition of amyloid precursor protein hyperphosphorylation and β-secretase activity, whereas simultaneous inhibition of PP2A abolished the antiamyloidogenic effects of I2(PP2A) silencing. Finally, silencing I2(PP2A) could improve learning and memory of tg2576 mice with preservation of several memory-associated components. Our data reveal that targeting I2(PP2A) can efficiently rescue Aβ toxicities and improve the memory deficits in tg2576 mice, suggesting that I2(PP2A) could be a promising target for potential AD therapies.
Figures

Similar articles
-
Cytoplasmic retention of protein phosphatase 2A inhibitor 2 (I2PP2A) induces Alzheimer-like abnormal hyperphosphorylation of Tau.J Biol Chem. 2014 Oct 3;289(40):27677-91. doi: 10.1074/jbc.M114.565358. Epub 2014 Aug 15. J Biol Chem. 2014. PMID: 25128526 Free PMC article.
-
CIP2A Causes Tau/APP Phosphorylation, Synaptopathy, and Memory Deficits in Alzheimer's Disease.Cell Rep. 2018 Jul 17;24(3):713-723. doi: 10.1016/j.celrep.2018.06.009. Cell Rep. 2018. PMID: 30021167 Free PMC article.
-
Alzheimer disease and amyotrophic lateral sclerosis: an etiopathogenic connection.Acta Neuropathol. 2014 Feb;127(2):243-56. doi: 10.1007/s00401-013-1175-9. Epub 2013 Oct 18. Acta Neuropathol. 2014. PMID: 24136402 Free PMC article.
-
Novel therapeutic strategies for neurodegenerative disease.Psychogeriatrics. 2009 Jun;9(2):103-9. doi: 10.1111/j.1479-8301.2009.00289.x. Psychogeriatrics. 2009. PMID: 19604333 Free PMC article. Review.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Cyclin-Dependent kinase 5 targeting prevents β-Amyloid aggregation involving glycogen synthase kinase 3β and phosphatases.J Neurosci Res. 2015 Aug;93(8):1258-66. doi: 10.1002/jnr.23576. Epub 2015 Feb 24. J Neurosci Res. 2015. PMID: 25711385 Free PMC article.
-
Adiponectin as a potential mediator of the pro-cognitive effects of physical exercise on Alzheimer's disease.Neural Regen Res. 2026 Jan 1;21(1):96-106. doi: 10.4103/NRR.NRR-D-23-00943. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885660 Free PMC article.
-
Epigenetic regulation of estrogen-dependent memory.Front Neuroendocrinol. 2014 Oct;35(4):530-49. doi: 10.1016/j.yfrne.2014.05.001. Epub 2014 May 28. Front Neuroendocrinol. 2014. PMID: 24878494 Free PMC article. Review.
-
Thirty years of SET/TAF1β/I2PP2A: from the identification of the biological functions to its implications in cancer and Alzheimer's disease.Biosci Rep. 2022 Nov 30;42(11):BSR20221280. doi: 10.1042/BSR20221280. Biosci Rep. 2022. PMID: 36345878 Free PMC article. Review.
-
Nature of Tau-Associated Neurodegeneration and the Molecular Mechanisms.J Alzheimers Dis. 2018;62(3):1305-1317. doi: 10.3233/JAD-170788. J Alzheimers Dis. 2018. PMID: 29562535 Free PMC article. Review.
References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. - PubMed
-
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. - PubMed
-
- Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. - PubMed
-
- Sun L, Liu SY, Zhou XW, Wang XC, Liu R, Wang Q, et al. Inhibition of protein phosphatase 2A- and protein phosphatase 1-induced tau hyperphosphorylation and impairment of spatial memory retention in rats. Neuroscience. 2003;118:1175–1182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

