Toxicology and Biodistribution Studies for MGH2.1, an Oncolytic Virus that Expresses Two Prodrug-activating Genes, in Combination with Prodrugs
- PMID: 23922029
- PMCID: PMC3759737
- DOI: 10.1038/mtna.2013.38
Toxicology and Biodistribution Studies for MGH2.1, an Oncolytic Virus that Expresses Two Prodrug-activating Genes, in Combination with Prodrugs
Abstract
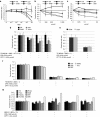
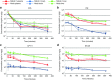
MGH2.1 is a herpes simplex virus type 1 (HSV1) oncolytic virus that expresses two prodrug-activating transgenes: the cyclophosphamide (CPA)-activating cytochrome P4502B1 (CYP2B1) and the CPT11-activating secreted human intestinal carboxylesterase (shiCE). Toxicology and biodistribution of MGH2.1 in the presence/absence of prodrugs was evaluated in mice. MGH2.1 ± prodrugs was cytotoxic to human glioma cells, but not to normal cells. Pharmacokinetically, intracranial MGH2.1 did not significantly alter the metabolism of intraperitoneally (i.p.) administered prodrugs in mouse plasma, brain, or liver. MGH2.1 did not induce an acute inflammatory reaction. MGH2.1 DNA was detected in brains of mice inoculated with 10(8) pfus for up to 60 days. However, only one animal showed evidence of viral gene expression at this time. Expression of virally encoded genes was restricted to brain. Intracranial inoculation of MGH2.1 did not induce lethality at 10(8) pfus in the absence of prodrugs and at 10(6) pfus in the presence of prodrugs. This study provides safety and toxicology data justifying a possible clinical trial of intratumoral injection of MGH2.1 with peripheral administration of CPA and/or CPT11 prodrugs in humans with malignant gliomas.Molecular Therapy-Nucleic Acids (2013) 2, e113; doi:10.1038/mtna.2013.38; published online 6 August 2013.
Figures


Similar articles
-
Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan.Cancer Res. 2005 Aug 1;65(15):6850-7. doi: 10.1158/0008-5472.CAN-05-0154. Cancer Res. 2005. PMID: 16061668
-
Prodrug bioactivation and oncolysis of diffuse liver metastases by a herpes simplex virus 1 mutant that expresses the CYP2B1 transgene.Cancer. 2002 Sep 1;95(5):1171-81. doi: 10.1002/cncr.10776. Cancer. 2002. PMID: 12209705
-
Intratumoral activation of cyclophosphamide by retroviral transfer of the cytochrome P450 2B1 in a pancreatic tumor model. Combination with the HSVtk/GCV system.J Gene Med. 2002 Mar-Apr;4(2):141-9. doi: 10.1002/jgm.247. J Gene Med. 2002. PMID: 11933215
-
Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer.Curr Pharm Des. 2002;8(15):1405-16. doi: 10.2174/1381612023394566. Curr Pharm Des. 2002. PMID: 12052216 Review.
-
Activation of oxazaphosphorines by cytochrome P450: application to gene-directed enzyme prodrug therapy for cancer.Toxicol In Vitro. 2006 Mar;20(2):176-86. doi: 10.1016/j.tiv.2005.06.046. Epub 2005 Nov 15. Toxicol In Vitro. 2006. PMID: 16293390 Review.
Cited by
-
Unlocking the promise of oncolytic virotherapy in glioma: combination with chemotherapy to enhance efficacy.Ther Deliv. 2015;6(4):453-68. doi: 10.4155/tde.14.123. Ther Deliv. 2015. PMID: 25996044 Free PMC article. Review.
-
Gene therapy for the nervous system: challenges and new strategies.Neurotherapeutics. 2014 Oct;11(4):817-39. doi: 10.1007/s13311-014-0299-5. Neurotherapeutics. 2014. PMID: 25159276 Free PMC article. Review.
-
Effect of Nicotine on CYP2B1 Expression in a Glioma Animal Model and Analysis of CYP2B6 Expression in Pediatric Gliomas.Int J Mol Sci. 2018 Jun 16;19(6):1790. doi: 10.3390/ijms19061790. Int J Mol Sci. 2018. PMID: 29914177 Free PMC article.
-
DDMC-p53 gene therapy with or without cisplatin and microwave ablation.Onco Targets Ther. 2015 May 20;8:1165-73. doi: 10.2147/OTT.S83794. eCollection 2015. Onco Targets Ther. 2015. PMID: 26056480 Free PMC article.
-
The Current State of Oncolytic Herpes Simplex Virus for Glioblastoma Treatment.Oncolytic Virother. 2021 Feb 24;10:1-27. doi: 10.2147/OV.S268426. eCollection 2021. Oncolytic Virother. 2021. PMID: 33659221 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Quick A, Patel D, Hadziahmetovic M, Chakravarti A, Mehta M. Current therapeutic paradigms in glioblastoma. Rev Recent Clin Trials. 2010;5:14–27. - PubMed
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

