Calcium-dependent protein kinase CPK6 positively functions in induction by yeast elicitor of stomatal closure and inhibition by yeast elicitor of light-induced stomatal opening in Arabidopsis
- PMID: 23922271
- PMCID: PMC3793040
- DOI: 10.1104/pp.113.224055
Calcium-dependent protein kinase CPK6 positively functions in induction by yeast elicitor of stomatal closure and inhibition by yeast elicitor of light-induced stomatal opening in Arabidopsis
Abstract
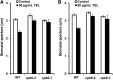
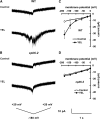
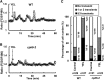
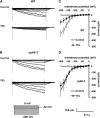
Yeast elicitor (YEL) induces stomatal closure that is mediated by a Ca(2+)-dependent signaling pathway. A Ca(2+)-dependent protein kinase, CPK6, positively regulates activation of ion channels in abscisic acid and methyl jasmonate signaling, leading to stomatal closure in Arabidopsis (Arabidopsis thaliana). YEL also inhibits light-induced stomatal opening. However, it remains unknown whether CPK6 is involved in induction by YEL of stomatal closure or in inhibition by YEL of light-induced stomatal opening. In this study, we investigated the roles of CPK6 in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening in Arabidopsis. Disruption of CPK6 gene impaired induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening. Activation by YEL of nonselective Ca(2+)-permeable cation channels was impaired in cpk6-2 guard cells, and transient elevations elicited by YEL in cytosolic-free Ca(2+) concentration were suppressed in cpk6-2 and cpk6-1 guard cells. YEL activated slow anion channels in wild-type guard cells but not in cpk6-2 or cpk6-1 and inhibited inward-rectifying K(+) channels in wild-type guard cells but not in cpk6-2 or cpk6-1. The cpk6-2 and cpk6-1 mutations inhibited YEL-induced hydrogen peroxide accumulation in guard cells and apoplast of rosette leaves but did not affect YEL-induced hydrogen peroxide production in the apoplast of rosette leaves. These results suggest that CPK6 positively functions in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening in Arabidopsis and is a convergent point of signaling pathways for stomatal closure in response to abiotic and biotic stress.
Figures

References
-
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 - PubMed
-
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 - PMC - PubMed
-
- Geiger D, Maierhofer T, Al-Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4: ra32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

