T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance
- PMID: 23922396
- PMCID: PMC3752276
- DOI: 10.1073/pnas.1312348110
T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance
Abstract
IgG2a is known to be the most efficient antibody isotype for viral clearance. Here, we demonstrate a unique pathway of B-cell activation, leading to IgG2a production, and involving synergistic stimulation via B-cell antigen receptors, toll-like receptor 7 (TLR7), and IFNγ receptors on B cells. This synergistic stimulation leads to induction of T-box transcription factor T-bet expression in B cells, which, in turn, drives expression of CD11b and CD11c on B cells. T-bet/CD11b/CD11c positive B cells appear during antiviral responses and produce high titers of antiviral IgG2a antibodies that are critical for efficient viral clearance. The results thus demonstrate a previously unknown role for T-bet expression in B cells during viral infections. Moreover, the appearance of T-bet(+) B cells during antiviral responses and during autoimmunity suggests a possible link between these two processes.
Keywords: interferon gamma; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


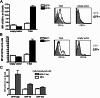

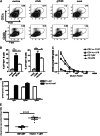
References
-
- Schmitz N, et al. Universal vaccine against influenza virus: Linking TLR signaling to anti-viral protection. Eur J Immunol. 2012;42(4):863–869. - PubMed
-
- Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236(4804):944–947. - PubMed
-
- Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int Immunol. 2003;15(8):937–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

