Indacaterol: a comprehensive review
- PMID: 23922496
- PMCID: PMC3728154
- DOI: 10.2147/COPD.S21625
Indacaterol: a comprehensive review
Abstract
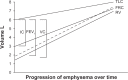
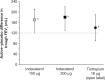
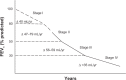
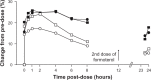
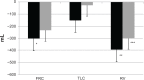
At present there is no cure for chronic obstructive pulmonary disease (COPD). However, some nonpharmacologic treatments, such as rehabilitation and lung volume reduction surgery, as well as pharmacologic intervention, can relieve some of the patient's symptoms and improve quality of life, while also reducing the rate of exacerbations and hospitalizations. There needs to be a paradigm shift away from the unjustified nihilistic approach to COPD towards considering it a preventable and treatable disease. After patients quit smoking and start to lead healthier lifestyles, long-acting bronchodilators, such as long-acting beta-adrenergic agents (LABA) and long-acting antimuscarinic agents (LAMA), are recommended as the cornerstone of treatment for COPD, either as monotherapy or in combination. COPD is characterized by a reduced maximum expiratory flow and slow forced emptying of the lungs, which progress over time and are not completely reversible. In this condition, gas gets trapped in the lungs and pulmonary hyperinflation occurs. LABA and LAMA improve airway patency and deflate the lungs. Indacaterol is the first once-daily LABA approved for treatment of COPD, and is administered by inhalation through the Breezhaler® device. The speed of bronchodilation is similar to that with salbutamol (ie, about five minutes) and longer (ie, 24 hours) than that with traditional LABA, with the same 12-hour effect as salmeterol and formoterol, both of which require twice-daily administration. This is why indacaterol has been called the "ultra-LABA". On the one hand, the fast onset of action provides immediate relief of symptoms, and on the other, its constant 24-hour bronchodilation provides "pharmacologic stenting" which facilitates lung emptying, thereby decreasing trapped gas and pulmonary hyperinflation. Once-daily administration of a fast and long-acting bronchodilator can improve patient adherence with therapy, which is known to be a major problem for many medical treatments. Dose-finding trials have shown that 75 μg is the minimum dose needed to achieve clinically important improvement. However, indacaterol 150 μg and 300 μg achieve an even greater improvement in lung function and patient-oriented outcomes. Further, these two doses of indacaterol significantly reduce pulmonary hyperinflation, thereby improving exercise tolerance and ability to perform day-to-day activities. It is more effective on lung volumes at the 300 μg dose than formoterol, and better than salmeterol and tiotropium at the 150 μg dose, at least in the acute setting. It is noteworthy that few studies document these results in patients with COPD and moderate airflow obstruction. These are exactly the kind of patients our research should be concentrating on, in view of the accelerated decay in forced expiratory volume in one second at this stage of the disease. Finally, all the relevant studies show that indacaterol is consistently well tolerated by patients with COPD at every stage, and that it has a high safety profile.
Keywords: chronic obstructive pulmonary disease; indacaterol.
Figures
Similar articles
-
Indacaterol: a review of its use as maintenance therapy in patients with chronic obstructive pulmonary disease.Drugs. 2012 Mar 5;72(4):543-63. doi: 10.2165/11208490-000000000-00000. Drugs. 2012. PMID: 22356291 Review.
-
Comparative efficacy of indacaterol in chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2012;7:145-52. doi: 10.2147/COPD.S19805. Epub 2012 Mar 5. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 22419862 Free PMC article. Review.
-
Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD.Thorax. 2010 Jun;65(6):473-9. doi: 10.1136/thx.2009.125435. Thorax. 2010. PMID: 20522841 Clinical Trial.
-
Clinical role of dual bronchodilation with an indacaterol-glycopyrronium combination in the management of COPD: its impact on patient-related outcomes and quality of life.Int J Chron Obstruct Pulmon Dis. 2015 Jul 23;10:1383-92. doi: 10.2147/COPD.S55488. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26229457 Free PMC article. Review.
-
Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial.Lancet Respir Med. 2018 May;6(5):368-378. doi: 10.1016/S2213-2600(18)30054-7. Epub 2018 Feb 21. Lancet Respir Med. 2018. PMID: 29477448 Clinical Trial.
Cited by
-
Optimizing Treatment of Elderly COPD Patients: What Role for Inhaled Corticosteroids?Drugs Aging. 2015 Sep;32(9):679-87. doi: 10.1007/s40266-015-0291-8. Drugs Aging. 2015. PMID: 26297533 Review.
-
Bronchodilators for hyperinflation in COPD associated with biomass smoke: clinical trial.Int J Chron Obstruct Pulmon Dis. 2019 Aug 6;14:1753-1762. doi: 10.2147/COPD.S201314. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31496674 Free PMC article. Clinical Trial.
-
Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan.Int J Chron Obstruct Pulmon Dis. 2015 Apr 21;10:813-22. doi: 10.2147/COPD.S56067. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25960646 Free PMC article. Review.
-
Pharmacologic Activities of Plant-Derived Natural Products on Respiratory Diseases and Inflammations.Biomed Res Int. 2021 Oct 4;2021:1636816. doi: 10.1155/2021/1636816. eCollection 2021. Biomed Res Int. 2021. PMID: 34646882 Free PMC article. Review.
-
The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro.Mediators Inflamm. 2014;2014:105420. doi: 10.1155/2014/105420. Epub 2014 Mar 6. Mediators Inflamm. 2014. PMID: 24733958 Free PMC article.
References
-
- Hanania NA, Marciniuk DD. A unified front against COPD: clinical practice guidelines from the American College of Physicians, the American College of Chest Physicians, the American Thoracic Society, and the European Society. Chest. 2011;140(3):565–566. - PubMed
-
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675–679. - PubMed
-
- Maltais F, Dennis N, Chan CK. Rationale for earlier treatment in COPD: a systematic review of published literature in mild-to-moderate COPD. COPD. 2013;10(1):79–103. - PubMed
-
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793. - PubMed
-
- Egan C, Deering BM, Blake C, et al. Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med. 2012;106(12):1671–1679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

