Spatial relationships between GABAergic and glutamatergic synapses on the dendrites of distinct types of mouse retinal ganglion cells across development
- PMID: 23922756
- PMCID: PMC3724919
- DOI: 10.1371/journal.pone.0069612
Spatial relationships between GABAergic and glutamatergic synapses on the dendrites of distinct types of mouse retinal ganglion cells across development
Abstract
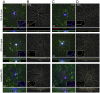
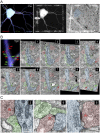
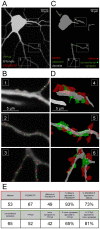
Neuronal output requires a concerted balance between excitatory and inhibitory (I/E) input. Like other circuits, inhibitory synaptogenesis in the retina precedes excitatory synaptogenesis. How then do neurons attain their mature balance of I/E ratios despite temporal offset in synaptogenesis? To directly compare the development of glutamatergic and GABAergic synapses onto the same cell, we biolistically transfected retinal ganglion cells (RGCs) with PSD95CFP, a marker of glutamatergic postsynaptic sites, in transgenic Thy1-YFPγ2 mice in which GABAA receptors are fluorescently tagged. We mapped YFPγ2 and PSD95CFP puncta distributions on three RGC types at postnatal day P12, shortly before eye opening, and at P21 when robust light responses in RGCs are present. The mature IGABA/E ratios varied among ON-Sustained (S) A-type, OFF-S A-type, and bistratified direction selective (DS) RGCs. These ratios were attained at different rates, before eye-opening for ON-S and OFF-S A-type, and after eye-opening for DS RGCs. At both ages examined, the IGABA/E ratio was uniform across the arbors of the three RGC types. Furthermore, measurements of the distances between neighboring PSD95CFP and YFPγ2 puncta on RGC dendrites indicate that their local relationship is established early in development, and cannot be predicted by random organization. These close spatial associations between glutamatergic and GABAergic postsynaptic sites appear to represent local synaptic arrangements revealed by correlative light and EM reconstructions of a single RGC's dendrites. Thus, although RGC types have different IGABA/E ratios and establish these ratios at separate rates, the local relationship between excitatory and inhibitory inputs appear similarly constrained across the RGC types studied.
Conflict of interest statement
Figures






References
-
- Jia H, Rochefort NL, Chen X, Konnerth A (2010) Dendritic organization of sensory input to cortical neurons in vivo. Nature 464: 1307–1312. - PubMed
-
- Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A (2011) Functional mapping of single spines in cortical neurons in vivo. Nature. - PubMed
-
- Megias M, Emri ZS, Freund TF, Gulyas AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102: 527–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

