Schiff base switch II precedes the retinal thermal isomerization in the photocycle of bacteriorhodopsin
- PMID: 23922839
- PMCID: PMC3726731
- DOI: 10.1371/journal.pone.0069882
Schiff base switch II precedes the retinal thermal isomerization in the photocycle of bacteriorhodopsin
Abstract
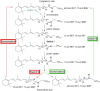
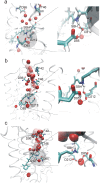
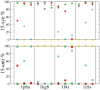
In bacteriorhodopsin, the order of molecular events that control the cytoplasmic or extracellular accessibility of the Schiff bases (SB) are not well understood. We use molecular dynamics simulations to study a process involved in the second accessibility switch of SB that occurs after its reprotonation in the N intermediate of the photocycle. We find that once protonated, the SB C15 = NZ bond switches from a cytoplasmic facing (13-cis, 15-anti) configuration to an extracellular facing (13-cis, 15-syn) configuration on the pico to nanosecond timescale. Significantly, rotation about the retinal's C13 = C14 double bond is not observed. The dynamics of the isomeric state transitions of the protonated SB are strongly influenced by the surrounding charges and dielectric effects of other buried ions, particularly D96 and D212. Our simulations indicate that the thermal isomerization of retinal from 13-cis back to all-trans likely occurs independently from and after the SB C15 = NZ rotation in the N-to-O transition.
Conflict of interest statement
Figures


References
-
- Oesterhelt D, Stoeckenius W (1971) Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol 233: 149–152. - PubMed
-
- Haupts U, Tittor J, Oesterhelt D (1999) Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu Rev Biophys Biomol Struct 28: 367–399. - PubMed
-
- Heberle J (2000) Proton transfer reactions across bacteriorhodopsin and along the membrane. BBA Bioenergetics 1458: 135–147. - PubMed
-
- Lanyi JK (2004) Bacteriorhodopsin. Annu Rev Physiol 66: 665–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

