Persistent posttetanic depression at cerebellar parallel fiber to Purkinje cell synapses
- PMID: 23922966
- PMCID: PMC3726549
- DOI: 10.1371/journal.pone.0070277
Persistent posttetanic depression at cerebellar parallel fiber to Purkinje cell synapses
Abstract
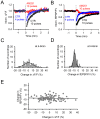
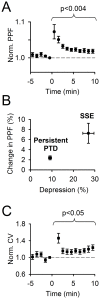
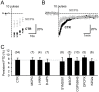
Plasticity at the cerebellar parallel fiber to Purkinje cell synapse may underlie information processing and motor learning. In vivo, parallel fibers appear to fire in short high frequency bursts likely to activate sparsely distributed synapses over the Purkinje cell dendritic tree. Here, we report that short parallel fiber tetanic stimulation evokes a ∼7-15% depression which develops over 2 min and lasts for at least 20 min. In contrast to the concomitantly evoked short-term endocannabinoid-mediated depression, this persistent posttetanic depression (PTD) does not exhibit a dependency on the spatial pattern of synapse activation and is not caused by any detectable change in presynaptic calcium signaling. This persistent PTD is however associated with increased paired-pulse facilitation and coefficient of variation of synaptic responses, suggesting that its expression is presynaptic. The chelation of postsynaptic calcium prevents its induction, suggesting that post- to presynaptic (retrograde) signaling is required. We rule out endocannabinoid signaling since the inhibition of type 1 cannabinoid receptors, monoacylglycerol lipase or vanilloid receptor 1, or incubation with anandamide had no detectable effect. The persistent PTD is maximal in pre-adolescent mice, abolished by adrenergic and dopaminergic receptors block, but unaffected by adrenergic and dopaminergic agonists. Our data unveils a novel form of plasticity at parallel fiber synapses: a persistent PTD induced by physiologically relevant input patterns, age-dependent, and strongly modulated by the monoaminergic system. We further provide evidence supporting that the plasticity mechanism involves retrograde signaling and presynaptic diacylglycerol.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

