Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice
- PMID: 23922988
- PMCID: PMC3724941
- DOI: 10.1371/journal.pone.0070409
Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice
Abstract
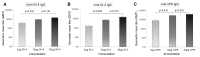
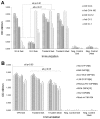
Rotavirus (RV) and norovirus (NoV) are the two major causes of viral gastroenteritis (GE) in children worldwide. We have developed an injectable vaccine design to prevent infection or GE induced with these enteric viruses. The trivalent combination vaccine consists of NoV capsid (VP1) derived virus-like particles (VLPs) of GI-3 and GII-4 representing the two major NoV genogroups and tubular RV recombinant VP6 (rVP6), the most conserved and abundant RV protein. Each component was produced in insect cells by a recombinant baculovirus expression system and combined in vitro. The vaccine components were administered intramuscularly to BALB/c mice either separately or in the trivalent combination. High levels of NoV and RV type specific serum IgGs with high avidity (>50%) as well as intestinal IgGs were detected in the immunized mice. Cross-reactive IgG antibodies were also elicited against heterologous NoV VLPs not used for immunization (GII-4 NO, GII-12 and GI-1 VLPs) and to different RVs from cell cultures. NoV-specific serum antibodies blocked binding of homologous and heterologous VLPs to the putative receptors, histo-blood group antigens, suggesting broad NoV neutralizing activity of the sera. Mucosal antibodies of mice immunized with the trivalent combination vaccine inhibited RV infection in vitro. In addition, cross-reactive T cell immune responses to NoV and RV-specific antigens were detected. All the responses were sustained for up to six months. No mutual inhibition of the components in the trivalent vaccine combination was observed. In conclusion, the NoV GI and GII VLPs combination induced broader cross-reactive and potentially neutralizing immune responses than either of the VLPs alone. Therefore, trivalent vaccine might induce protective immune responses to the vast majority of circulating NoV and RV genotypes.
Conflict of interest statement
Figures




References
-
- Dennehy PH (2011) Viral gastroenteritis in children. Pediatr Infect Dis J 30: 63-64. doi:10.1097/INF.0b013e31820ad2ba. PubMed: 21173676. - DOI - PubMed
-
- Bucardo F, Lindgren PE, Svensson L, Nordgren J (2011) Low prevalence of rotavirus and high prevalence of norovirus in hospital and community wastewater after introduction of rotavirus vaccine in nicaragua. PLOS ONE 6: e25962. doi:10.1371/journal.pone.0025962. PubMed: 22016794. - DOI - PMC - PubMed
-
- Puustinen L, Blazevic V, Salminen M, Hämäläinen M, Räsänen S et al. (2011) Noroviruses as a major cause of acute gastroenteritis in children in finland, 2009-2010. Scand J Infect Dis 43: 804-808. doi:10.3109/00365548.2011.588610. PubMed: 21696253. - DOI - PubMed
-
- Hemming M, Rasanen S, Huhti L, Paloniemi M, Salminen M et al. (2013) Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the national immunization programme in Finland. Eur J Pediatr. doi:10.1007/s00431-013-1945-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

