Relationship between the magnitude of intraocular pressure during an episode of acute elevation and retinal damage four weeks later in rats
- PMID: 23922999
- PMCID: PMC3726657
- DOI: 10.1371/journal.pone.0070513
Relationship between the magnitude of intraocular pressure during an episode of acute elevation and retinal damage four weeks later in rats
Abstract
Purpose: To determine relationship between the magnitude of intraocular pressure (IOP) during a fixed-duration episode of acute elevation and the loss of retinal function and structure 4 weeks later in rats.
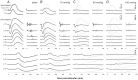
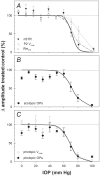
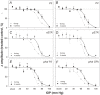
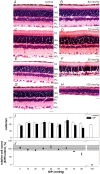
Methods: Unilateral elevation of IOP (105 minutes) was achieved manometrically in adult Brown Norway rats (9 groups; n = 4 to 8 each, 10-100 mm Hg and sham control). Full-field ERGs were recorded simultaneously from treated and control eyes 4 weeks after IOP elevation. Scotopic ERG stimuli were white flashes (-6.04 to 2.72 log cd.s.m(-2)). Photopic ERGs were recorded (1.22 to 2.72 log cd.s.m(-2)) after 15 min of light adaptation (150 cd/m(2)). Relative amplitude (treated/control, %) of ERG components versus IOP was described with a cummulative normal function. Retinal ganglion cell (RGC) layer density was determined post mortem by histology.
Results: All ERG components failed to recover completely normal amplitudes by 4 weeks after the insult if IOP was 70 mmHg or greater during the episode. There was no ERG recovery at all if IOP was 100 mmHg. Outer retinal (photoreceptor) function demonstrated the least sensitivity to prior acute IOP elevation. ERG components reflecting inner retinal function were correlated with post mortem RGC layer density.
Conclusions: Retinal function recovers after IOP normalization, such that it requires a level of acute IOP elevation approximately 10 mmHg higher to cause a pattern of permanent dysfunction similar to that observed during the acute event. There is a 'threshold' for permanent retinal functional loss in the rat at an IOP between 60 and 70 mmHg if sustained for 105 minutes or more.
Conflict of interest statement
Figures


References
-
- Goldblum D, Mittag T (2002) Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res 42: 471–478. - PubMed
-
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, et al. (2005) Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Visual Neuroscience 22: 637–648. - PubMed
-
- Pang IH, Clark AF (2007) Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma 16: 483–505. - PubMed
-
- Morrison JC, Johnson E, Cepurna WO (2008) Rat models for glaucoma research. Prog Brain Res 173: 285–301. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

