Distinct functional interactions between actin isoforms and nonsarcomeric myosins
- PMID: 23923011
- PMCID: PMC3724804
- DOI: 10.1371/journal.pone.0070636
Distinct functional interactions between actin isoforms and nonsarcomeric myosins
Abstract
Despite their near sequence identity, actin isoforms cannot completely replace each other in vivo and show marked differences in their tissue-specific and subcellular localization. Little is known about isoform-specific differences in their interactions with myosin motors and other actin-binding proteins. Mammalian cytoplasmic β- and γ-actin interact with nonsarcomeric conventional myosins such as the members of the nonmuscle myosin-2 family and myosin-7A. These interactions support a wide range of cellular processes including cytokinesis, maintenance of cell polarity, cell adhesion, migration, and mechano-electrical transduction. To elucidate differences in the ability of isoactins to bind and stimulate the enzymatic activity of individual myosin isoforms, we characterized the interactions of human skeletal muscle α-actin, cytoplasmic β-actin, and cytoplasmic γ-actin with human myosin-7A and nonmuscle myosins-2A, -2B and -2C1. In the case of nonmuscle myosins-2A and -2B, the interaction with either cytoplasmic actin isoform results in 4-fold greater stimulation of myosin ATPase activity than was observed in the presence of α-skeletal muscle actin. Nonmuscle myosin-2C1 is most potently activated by β-actin and myosin-7A by γ-actin. Our results indicate that β- and γ-actin isoforms contribute to the modulation of nonmuscle myosin-2 and myosin-7A activity and thereby to the spatial and temporal regulation of cytoskeletal dynamics. FRET-based analyses show efficient copolymerization abilities for the actin isoforms in vitro. Experiments with hybrid actin filaments show that the extent of actomyosin coupling efficiency can be regulated by the isoform composition of actin filaments.
Conflict of interest statement
Figures
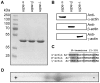

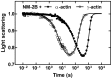
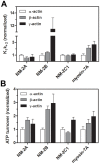
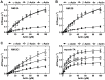
References
-
- Vandekerckhove J, Weber K (1978) At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol 126: 783–802. - PubMed
-
- Tondeleir D, Vandamme D, Vandekerckhove J, Ampe C, Lambrechts A (2009) Actin isoform expression patterns during mammalian development and in pathology: insights from mouse models. Cell Motil Cytoskeleton 66: 798–815. - PubMed
-
- Dugina V, Zwaenepoel I, Gabbiani G, Clement S, Chaponnier C (2009) Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci 122: 2980–2988. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

