Abundant intracellular IgG in enterocytes and endoderm lacking FcRn
- PMID: 23923029
- PMCID: PMC3726603
- DOI: 10.1371/journal.pone.0070863
Abundant intracellular IgG in enterocytes and endoderm lacking FcRn
Abstract
FcRn, a non-classical MHCI molecule, transports IgG from mother to young and regulates the rate of IgG degradation throughout life. Brambell proposed a mechanism that unified these two functions, saying that IgG was pinocytosed nonspecifically by the cell into an FcRn-expressing endosome, where, at low pH, it bound to FcRn and was exocytosed. This theory was immediately challenged by claims that FcRn specificity for ligand could be conferred at the cell surface in neonatal jejunum. Assessing Brambell's hypothesis we found abundant nonspecifically endocytosed IgG present in the cytoplasm of FcRn(-/-) enterocytes. Further, IgG was present in the intercellular clefts and the cores of FcRn(+/+) but not FcRn(-/-) jejunum. FcRn specificity for ligand could be determined within the cell.
Conflict of interest statement
Figures

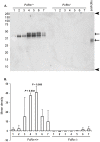

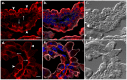
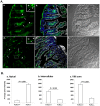

References
-
- Brambell FWR (1966) The transmission of immunity from mother to young and catabolism of immunoglobulins. Lancet ii: 1087–1093. - PubMed
-
- Brambell FWR (1970) The Transmission of Passive Immunity from Mother to Young. Amsterdam: North Holland Publishing Company. 385 p.
-
- Simister NE, Mostov KE (1989) An Fc receptor structurally related to MHC class I antigens. Nature. 337: 184–187. - PubMed
-
- Ahouse JJ, Hagerman CL, Mittal P, Gilbert DJ, Copeland NG, et al. (1993) Mouse MHC class I-like Fc receptor encoded outside the MHC. J Immunol. 151: 6076–6088. - PubMed
-
- Burmeister WP, Gastinel LN, Simister NE, Blum ML, Bjorkman PJ (1994) Crystal structure at 2.2 Å resolution of the MHC-related neonatal Fc receptor. Nature. 372: 336–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

