Rapid fabricating technique for multi-layered human hepatic cell sheets by forceful contraction of the fibroblast monolayer
- PMID: 23923035
- PMCID: PMC3724772
- DOI: 10.1371/journal.pone.0070970
Rapid fabricating technique for multi-layered human hepatic cell sheets by forceful contraction of the fibroblast monolayer
Abstract
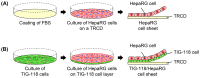
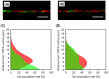
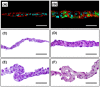
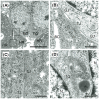
Cell sheet engineering is attracting attention from investigators in various fields, from basic research scientists to clinicians focused on regenerative medicine. However, hepatocytes have a limited proliferation potential in vitro, and it generally takes a several days to form a sheet morphology and multi-layered sheets. We herein report our rapid and efficient technique for generating multi-layered human hepatic cell (HepaRG® cell) sheets using pre-cultured fibroblast monolayers derived from human skin (TIG-118 cells) as a feeder layer on a temperature-responsive culture dish. Multi-layered TIG-118/HepaRG cell sheets with a thick morphology were harvested on day 4 of culturing HepaRG cells by forceful contraction of the TIG-118 cells, and the resulting sheet could be easily handled. In addition, the human albumin and alpha 1-antitrypsin synthesis activities of TIG-118/HepaRG cells were approximately 1.2 and 1.3 times higher than those of HepaRG cells, respectively. Therefore, this technique is considered to be a promising modality for rapidly fabricating multi-layered human hepatocyte sheets from cells with limited proliferation potential, and the engineered cell sheet could be used for cell transplantation with highly specific functions.
Conflict of interest statement
Figures


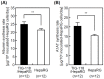
Similar articles
-
Controlled cell morphology and liver-specific function of engineered primary hepatocytes by fibroblast layer cell densities.J Biosci Bioeng. 2018 Aug;126(2):249-257. doi: 10.1016/j.jbiosc.2018.02.006. Epub 2018 Mar 5. J Biosci Bioeng. 2018. PMID: 29519653
-
Cell shape regulation based on hepatocyte sheet engineering technologies.Cell Transplant. 2012;21(2-3):411-20. doi: 10.3727/096368911X605312. Cell Transplant. 2012. PMID: 22793048
-
In vitro and in vivo fabrication of stable human hepatocyte tissue in combination with normal fibroblasts derived from donors of various ages.J Biosci Bioeng. 2019 Dec;128(6):766-772. doi: 10.1016/j.jbiosc.2019.05.009. Epub 2019 Jun 13. J Biosci Bioeng. 2019. PMID: 31202728
-
Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering.J Control Release. 2015 May 10;205:83-8. doi: 10.1016/j.jconrel.2014.12.016. Epub 2014 Dec 16. J Control Release. 2015. PMID: 25523520 Review.
-
Cell sheet-based tissue engineering for fabricating 3-dimensional heart tissues.Circ J. 2014;78(11):2594-603. doi: 10.1253/circj.cj-14-0973. Epub 2014 Oct 16. Circ J. 2014. PMID: 25319318 Review.
Cited by
-
Fundamental Technologies and Recent Advances of Cell-Sheet-Based Tissue Engineering.Int J Mol Sci. 2021 Jan 3;22(1):425. doi: 10.3390/ijms22010425. Int J Mol Sci. 2021. PMID: 33401626 Free PMC article. Review.
-
A novel approach for hepatocyte transplantation at the liver surface.Cell Transplant. 2025 Jan-Dec;34:9636897251329308. doi: 10.1177/09636897251329308. Epub 2025 Apr 10. Cell Transplant. 2025. PMID: 40208805 Free PMC article.
-
Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice.Sci Rep. 2015 Nov 10;5:16169. doi: 10.1038/srep16169. Sci Rep. 2015. PMID: 26553591 Free PMC article.
-
Rapid production of engineered human primary hepatocyte/fibroblast sheets.Data Brief. 2015 Oct 9;5:498-501. doi: 10.1016/j.dib.2015.09.044. eCollection 2015 Dec. Data Brief. 2015. PMID: 26629493 Free PMC article.
-
Hepatocyte Transplantation: Cell Sheet Technology for Liver Cell Transplantation.Curr Transplant Rep. 2017;4(3):184-192. doi: 10.1007/s40472-017-0156-7. Epub 2017 Aug 8. Curr Transplant Rep. 2017. PMID: 28932649 Free PMC article. Review.
References
-
- Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, et al. (2012) Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc 7: 850–858. - PubMed
-
- Ito A, Jitsunobu H, Kawabe Y, Kamihira M (2007) Construction of heterotypic cell sheets by magnetic force-based 3-D coculture of HepG2 and NIH3T3 cells. J Biosci Bioeng 104: 371–378. - PubMed
-
- Kino-oka M, Ngo TX, Nagamori E, Takezawa Y, Miyake Y, et al. (2012) Evaluation of vertical cell fluidity in a multilayered sheet of skeletal myoblasts. J Biosci Bioeng 113: 128–131. - PubMed
-
- Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, et al. (2010) Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 31: 1646–1654. - PubMed
-
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, et al. (2004) Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351: 1187–1196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

