In vivo administration of a JAK3 inhibitor to chronically siv infected rhesus macaques leads to NK cell depletion associated with transient modest increase in viral loads
- PMID: 23923040
- PMCID: PMC3724739
- DOI: 10.1371/journal.pone.0070992
In vivo administration of a JAK3 inhibitor to chronically siv infected rhesus macaques leads to NK cell depletion associated with transient modest increase in viral loads
Abstract
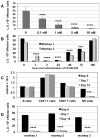
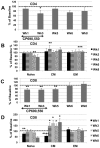
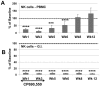
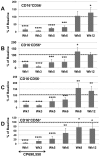
Innate immune responses are reasoned to play an important role during both acute and chronic SIV infection and play a deterministic role during the acute stages on the rate of infection and disease progression. NK cells are an integral part of the innate immune system but their role in influencing the course of SIV infection has been a subject of debate. As a means to delineate the effect of NK cells on SIV infection, use was made of a Janus kinase 3 (JAK3) inhibitor that has previously been shown to be effective in the depletion of NK cells in vivo in nonhuman primates (NHP). Extensive safety and in vitro/in vivo PK studies were conducted and an optimal dose that depletes NK cells and NK cell function in vivo identified. Six chronically SIV infected rhesus macaques, 3 with undetectable/low plasma viral loads and 3 with high plasma viral loads were administered a daily oral dose of 10 mg/kg for 35 days. Data obtained showed that, at the dose tested, the major cell lineage affected both in the blood and the GI tissues were the NK cells. Such depletion appeared to be associated with a transient increase in plasma and GI tissue viral loads. Whereas the number of NK cells returned to baseline values in the blood, the GI tissues remained depleted of NK cells for a prolonged period of time. Recent findings show that the JAK3 inhibitor utilized in the studies reported herein has a broader activity than previously reported with dose dependent effects on both JAK2 and JAK1 suggests that it is likely that multiple pathways are affected with the administration of this drug that needs to be taken into account. The findings reported herein are the first studies on the use of a JAK3 inhibitor in lentivirus infected NHP.
Conflict of interest statement
Figures
Similar articles
-
In vivo administration of a JAK3 inhibitor during acute SIV infection leads to significant increases in viral load during chronic infection.PLoS Pathog. 2014 Mar 6;10(3):e1003929. doi: 10.1371/journal.ppat.1003929. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24603870 Free PMC article.
-
Monkeying Around: Using Non-human Primate Models to Study NK Cell Biology in HIV Infections.Front Immunol. 2019 May 22;10:1124. doi: 10.3389/fimmu.2019.01124. eCollection 2019. Front Immunol. 2019. PMID: 31191520 Free PMC article. Review.
-
Functional Perturbation of Mucosal Group 3 Innate Lymphoid and Natural Killer Cells in Simian-Human Immunodeficiency Virus/Simian Immunodeficiency Virus-Infected Infant Rhesus Macaques.J Virol. 2020 Feb 14;94(5):e01644-19. doi: 10.1128/JVI.01644-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801861 Free PMC article.
-
Natural Killer Cells Regulate Acute SIV Replication, Dissemination, and Inflammation, but Do Not Impact Independent Transmission Events.J Virol. 2023 Jan 31;97(1):e0151922. doi: 10.1128/jvi.01519-22. Epub 2022 Dec 13. J Virol. 2023. PMID: 36511699 Free PMC article.
-
Role of follicular homing natural killer cells in HIV infection.Curr Opin HIV AIDS. 2025 Mar 1;20(2):154-158. doi: 10.1097/COH.0000000000000916. Epub 2025 Jan 2. Curr Opin HIV AIDS. 2025. PMID: 39773847 Review.
Cited by
-
Roles of natural killer cells in antiviral immunity.Curr Opin Virol. 2016 Feb;16:15-23. doi: 10.1016/j.coviro.2015.10.008. Epub 2015 Nov 16. Curr Opin Virol. 2016. PMID: 26590692 Free PMC article. Review.
-
In vivo administration of a JAK3 inhibitor during acute SIV infection leads to significant increases in viral load during chronic infection.PLoS Pathog. 2014 Mar 6;10(3):e1003929. doi: 10.1371/journal.ppat.1003929. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24603870 Free PMC article.
-
Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro.Antimicrob Agents Chemother. 2014;58(4):1977-86. doi: 10.1128/AAC.02496-13. Epub 2014 Jan 13. Antimicrob Agents Chemother. 2014. PMID: 24419350 Free PMC article.
-
NK cells modulate in vivo control of SARS-CoV-2 replication and suppression of lung damage.PLoS Pathog. 2024 Aug 12;20(8):e1012439. doi: 10.1371/journal.ppat.1012439. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39133756 Free PMC article.
-
Monkeying Around: Using Non-human Primate Models to Study NK Cell Biology in HIV Infections.Front Immunol. 2019 May 22;10:1124. doi: 10.3389/fimmu.2019.01124. eCollection 2019. Front Immunol. 2019. PMID: 31191520 Free PMC article. Review.
References
-
- Ansari AA, Mayne AE, Takahashi Y, Pattanapanyasat K (2011) Incorporation of innate immune effector mechanisms in the formulation of a vaccine against HIV-1. Adv Exp Med Biol 780: 143–159. - PubMed
-
- Centlivre M, Sala M, Wain-Hobson S, Berkhout B (2007) In HIV-1 pathogenesis the die is cast during primary infection. AIDS 21: 1–11. - PubMed
-
- Haase AT (2010) Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464: 217–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

