ß-blocker timolol prevents arrhythmogenic Ca²⁺ release and normalizes Ca²⁺ and Zn²⁺ dyshomeostasis in hyperglycemic rat heart
- PMID: 23923043
- PMCID: PMC3726605
- DOI: 10.1371/journal.pone.0071014
ß-blocker timolol prevents arrhythmogenic Ca²⁺ release and normalizes Ca²⁺ and Zn²⁺ dyshomeostasis in hyperglycemic rat heart
Abstract
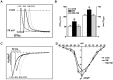
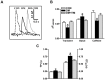
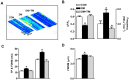
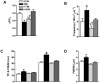
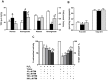
Defective cardiac mechanical activity in diabetes results from alterations in intracellular Ca(2+) handling, in part, due to increased oxidative stress. Beta-blockers demonstrate marked beneficial effects in heart dysfunction with scavenging free radicals and/or acting as an antioxidant. The aim of this study was to address how β-blocker timolol-treatment of diabetic rats exerts cardioprotection. Timolol-treatment (12-week), one-week following diabetes induction, prevented diabetes-induced depressed left ventricular basal contractile activity, prolonged cellular electrical activity, and attenuated the increase in isolated-cardiomyocyte size without hyperglycemic effect. Both in vivo and in vitro timolol-treatment of diabetic cardiomyocytes prevented the altered kinetic parameters of Ca(2+) transients and reduced Ca(2+) loading of sarcoplasmic reticulum (SR), basal intracellular free Ca(2+) and Zn(2+) ([Ca(2+)]i and [Zn(2+)]i), and spatio-temporal properties of the Ca(2+) sparks, significantly. Timolol also antagonized hyperphosphorylation of cardiac ryanodine receptor (RyR2), and significantly restored depleted protein levels of both RyR2 and calstabin2. Western blot analysis demonstrated that timolol-treatment also significantly normalized depressed levels of some [Ca(2+)]i-handling regulators, such as Na(+)/Ca(2+) exchanger (NCX) and phospho-phospholamban (pPLN) to PLN ratio. Incubation of diabetic cardiomyocytes with 4-mM glutathione exerted similar beneficial effects on RyR2-macromolecular complex and basal levels of both [Ca(2+)]i and [Zn(2+)]i, increased intracellular Zn(2+) hyperphosphorylated RyR2 in a concentration-dependent manner. Timolol also led to a balanced oxidant/antioxidant level in both heart and circulation and prevented altered cellular redox state of the heart. We thus report, for the first time, that the preventing effect of timolol, directly targeting heart, seems to be associated with a normalization of macromolecular complex of RyR2 and some Ca(2+) handling regulators, and prevention of Ca(2+) leak, and thereby normalization of both [Ca(2+)]i and [Zn(2+)]i homeostasis in diabetic rat heart, at least in part by controlling the cellular redox status of hyperglycemic cardiomyocytes.
Conflict of interest statement
Figures

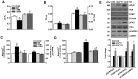

Similar articles
-
Enhancement of cellular antioxidant-defence preserves diastolic dysfunction via regulation of both diastolic Zn2+ and Ca2+ and prevention of RyR2-leak in hyperglycemic cardiomyocytes.Oxid Med Cell Longev. 2014;2014:290381. doi: 10.1155/2014/290381. Epub 2014 Feb 13. Oxid Med Cell Longev. 2014. PMID: 24693334 Free PMC article.
-
β-adrenergic receptor blockade reduces endoplasmic reticulum stress and normalizes calcium handling in a coronary embolization model of heart failure in canines.Cardiovasc Res. 2011 Aug 1;91(3):447-55. doi: 10.1093/cvr/cvr106. Epub 2011 Apr 14. Cardiovasc Res. 2011. PMID: 21493701
-
Profound cardioprotection with timolol in a female rat model of aging-related altered left ventricular function.Can J Physiol Pharmacol. 2011 Apr;89(4):277-88. doi: 10.1139/y11-018. Epub 2011 Apr 28. Can J Physiol Pharmacol. 2011. PMID: 21526975
-
A Comparative Summary on Antioxidant-like Actions of Timolol with Other Antioxidants in Diabetic Cardiomyopathy.Curr Drug Deliv. 2016;13(3):418-23. doi: 10.2174/1567201813666151123103354. Curr Drug Deliv. 2016. PMID: 26593438 Review.
-
Regulation of sarcoplasmic reticulum Ca2+ release by serine-threonine phosphatases in the heart.J Mol Cell Cardiol. 2016 Dec;101:156-164. doi: 10.1016/j.yjmcc.2016.08.020. Epub 2016 Aug 29. J Mol Cell Cardiol. 2016. PMID: 27585747 Free PMC article. Review.
Cited by
-
Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology.Int J Mol Sci. 2017 Nov 12;18(11):2395. doi: 10.3390/ijms18112395. Int J Mol Sci. 2017. PMID: 29137144 Free PMC article. Review.
-
Cardioprotective role of SIRT1 activation on mitochondrial function in insulin-resistant H9c2 cells.BMC Cardiovasc Disord. 2025 Mar 29;25(1):232. doi: 10.1186/s12872-024-04397-7. BMC Cardiovasc Disord. 2025. PMID: 40155821 Free PMC article.
-
Effect of intermittent hypoxia on the cardiac HIF-1/VEGF pathway in experimental type 1 diabetes mellitus.Anatol J Cardiol. 2016 Feb;16(2):76-83. doi: 10.5152/akd.2015.5925. Epub 2015 Apr 9. Anatol J Cardiol. 2016. PMID: 26467365 Free PMC article.
-
The Critical Roles of Zinc: Beyond Impact on Myocardial Signaling.Korean J Physiol Pharmacol. 2015 Sep;19(5):389-99. doi: 10.4196/kjpp.2015.19.5.389. Epub 2015 Aug 20. Korean J Physiol Pharmacol. 2015. PMID: 26330751 Free PMC article. Review.
-
The disappearance of IPO in myocardium of diabetes mellitus rats is associated with the increase of succinate dehydrogenase-flavin protein.BMC Cardiovasc Disord. 2021 Mar 17;21(1):142. doi: 10.1186/s12872-021-01949-z. BMC Cardiovasc Disord. 2021. PMID: 33731005 Free PMC article.
References
-
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, et al. (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602. - PubMed
-
- Fein FS, Kornstein LB, Strobeck JE, Capasso JM, Sonnenblick EH (1980) Altered myocardial mechanics in diabetic rats. Circ Res 47: 922–933. - PubMed
-
- Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, et al. (2005) Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes 54: 3082–3088. - PubMed
-
- Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS (1983) Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244: E528–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

