Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis
- PMID: 23924232
- PMCID: PMC3826269
- DOI: 10.1164/rccm.201212-2297OC
Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis
Abstract
Rationale: The origin of cells that make pathologic fibrillar collagen matrix in lung disease has been controversial. Recent studies suggest mesenchymal cells may contribute directly to fibrosis.
Objectives: To characterize discrete populations of mesenchymal cells in the normal mouse lung and to map their fate after bleomycin-induced lung injury.
Methods: We mapped the fate of Foxd1-expressing embryonic progenitors and their progeny during lung development, adult homeostasis, and after fibrosing injury in Foxd1-Cre; Rs26-tdTomato-R mice. We studied collagen-I(α)1-producing cells in normal and diseased lungs using Coll-GFP(Tg) mice.
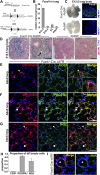
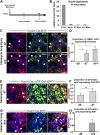
Measurements and main results: Foxd1-expressing embryonic progenitors enter lung buds before 13.5 days post-conception, expand, and form an extensive lineage of mesenchymal cells that have characteristics of pericytes. A collagen-I(α)1-expressing mesenchymal population of distinct lineage is also found in adult lung, with features of a resident fibroblast. In contrast to resident fibroblasts, Foxd1 progenitor-derived pericytes are enriched in transcripts for innate immunity, vascular development, WNT signaling pathway, and cell migration. Foxd1 progenitor-derived pericytes expand after bleomycin lung injury, and activate expression of collagen-I(α)1 and the myofibroblast marker αSMA in fibrotic foci. In addition, our studies suggest a distinct lineage of collagen-I(α)1-expressing resident fibroblasts that also expands after lung injury is a second major source of myofibroblasts.
Conclusions: We conclude that the lung contains an extensive population of Foxd1 progenitor-derived pericytes that are an important lung myofibroblast precursor population.
Figures




Comment in
-
New light is shed on the enigmatic origin of the lung myofibroblast.Am J Respir Crit Care Med. 2013 Oct 1;188(7):765-6. doi: 10.1164/rccm.201308-1494ED. Am J Respir Crit Care Med. 2013. PMID: 24083856 Free PMC article. No abstract available.
References
-
- Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–511. - PubMed
-
- Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. - PubMed
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. - PubMed
-
- Luppi F, Spagnolo P, Cerri S, Richeldi L. The big clinical trials in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:428–432. - PubMed
-
- Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UH3 TR000504/TR/NCATS NIH HHS/United States
- R01 DK093493/DK/NIDDK NIH HHS/United States
- TR000504/TR/NCATS NIH HHS/United States
- R01 HL086883/HL/NHLBI NIH HHS/United States
- DK094933/DK/NIDDK NIH HHS/United States
- DK84077/DK/NIDDK NIH HHS/United States
- R01 DK084077/DK/NIDDK NIH HHS/United States
- R24 DK094768/DK/NIDDK NIH HHS/United States
- DK87389/DK/NIDDK NIH HHS/United States
- K24 HL068796/HL/NHLBI NIH HHS/United States
- UH2 TR000504/TR/NCATS NIH HHS/United States
- RC1 DK087389/DK/NIDDK NIH HHS/United States
- DK93493/DK/NIDDK NIH HHS/United States
- DK94768/DK/NIDDK NIH HHS/United States
- R01 DK094933/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

