Insights into xanthomonas axonopodis pv. citri biofilm through proteomics
- PMID: 23924281
- PMCID: PMC3750573
- DOI: 10.1186/1471-2180-13-186
Insights into xanthomonas axonopodis pv. citri biofilm through proteomics
Abstract
Background: Xanthomonas axonopodis pv. citri (X. a. pv. citri) causes citrus canker that can result in defoliation and premature fruit drop with significant production losses worldwide. Biofilm formation is an important process in bacterial pathogens and several lines of evidence suggest that in X. a. pv. citri this process is a requirement to achieve maximal virulence since it has a major role in host interactions. In this study, proteomics was used to gain further insights into the functions of biofilms.
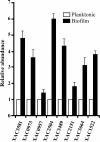
Results: In order to identify differentially expressed proteins, a comparative proteomic study using 2D difference gel electrophoresis was carried out on X. a. pv. citri mature biofilm and planktonic cells. The biofilm proteome showed major variations in the composition of outer membrane proteins and receptor or transport proteins. Among them, several porins and TonB-dependent receptor were differentially regulated in the biofilm compared to the planktonic cells, indicating that these proteins may serve in maintaining specific membrane-associated functions including signaling and cellular homeostasis. In biofilms, UDP-glucose dehydrogenase with a major role in exopolysaccharide production and the non-fimbrial adhesin YapH involved in adherence were over-expressed, while a polynucleotide phosphorylase that was demonstrated to negatively control biofilm formation in E. coli was down-regulated. In addition, several proteins involved in protein synthesis, folding and stabilization were up-regulated in biofilms. Interestingly, some proteins related to energy production, such as ATP-synthase were down-regulated in biofilms. Moreover, a number of enzymes of the tricarboxylic acid cycle were differentially expressed. In addition, X. a. pv. citri biofilms also showed down-regulation of several antioxidant enzymes. The respective gene expression patterns of several identified proteins in both X. a. pv. citri mature biofilm and planktonic cells were evaluated by quantitative real-time PCR and shown to consistently correlate with those deduced from the proteomic study.
Conclusions: Differentially expressed proteins are enriched in functional categories. Firstly, proteins that are down-regulated in X. a. pv. citri biofilms are enriched for the gene ontology (GO) terms 'generation of precursor metabolites and energy' and secondly, the biofilm proteome mainly changes in 'outer membrane and receptor or transport'. We argue that the differentially expressed proteins have a critical role in maintaining a functional external structure as well as enabling appropriate flow of nutrients and signals specific to the biofilm lifestyle.
Figures


Similar articles
-
Genetic and structural determinants on iron assimilation pathways in the plant pathogen Xanthomonas citri subsp. citri and Xanthomonas sp.Braz J Microbiol. 2020 Sep;51(3):1219-1231. doi: 10.1007/s42770-019-00207-x. Epub 2020 Aug 28. Braz J Microbiol. 2020. PMID: 31848911 Free PMC article. Review.
-
A novel two-component response regulator links rpf with biofilm formation and virulence of Xanthomonas axonopodis pv. citri.PLoS One. 2013 Apr 23;8(4):e62824. doi: 10.1371/journal.pone.0062824. Print 2013. PLoS One. 2013. PMID: 23626857 Free PMC article.
-
Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri.Mol Plant Microbe Interact. 2007 Oct;20(10):1222-30. doi: 10.1094/MPMI-20-10-1222. Mol Plant Microbe Interact. 2007. PMID: 17918624
-
A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization.PLoS One. 2012;7(6):e38226. doi: 10.1371/journal.pone.0038226. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675525 Free PMC article.
-
Bacteria causing important diseases of citrus utilise distinct modes of pathogenesis to attack a common host.Appl Microbiol Biotechnol. 2010 Jun;87(2):467-77. doi: 10.1007/s00253-010-2631-2. Epub 2010 May 7. Appl Microbiol Biotechnol. 2010. PMID: 20449739 Review.
Cited by
-
Proteomics dedicated to biofilmology: What have we learned from a decade of research?Med Microbiol Immunol. 2016 Feb;205(1):1-19. doi: 10.1007/s00430-015-0423-0. Epub 2015 Jun 12. Med Microbiol Immunol. 2016. PMID: 26068406 Review.
-
3-methylcrotonyl Coenzyme A (CoA) carboxylase complex is involved in the Xanthomonas citri subsp. citri lifestyle during citrus infection.PLoS One. 2018 Jun 7;13(6):e0198414. doi: 10.1371/journal.pone.0198414. eCollection 2018. PLoS One. 2018. PMID: 29879157 Free PMC article.
-
Xanthomonas citri subsp. citri and Xanthomonas arboricola pv. pruni: Comparative analysis of two pathogens producing similar symptoms in different host plants.PLoS One. 2019 Jul 18;14(7):e0219797. doi: 10.1371/journal.pone.0219797. eCollection 2019. PLoS One. 2019. PMID: 31318915 Free PMC article.
-
The type III protein secretion system contributes to Xanthomonas citri subsp. citri biofilm formation.BMC Microbiol. 2014 Apr 18;14:96. doi: 10.1186/1471-2180-14-96. BMC Microbiol. 2014. PMID: 24742141 Free PMC article.
-
Genetic and structural determinants on iron assimilation pathways in the plant pathogen Xanthomonas citri subsp. citri and Xanthomonas sp.Braz J Microbiol. 2020 Sep;51(3):1219-1231. doi: 10.1007/s42770-019-00207-x. Epub 2020 Aug 28. Braz J Microbiol. 2020. PMID: 31848911 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

