Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters
- PMID: 23924785
- PMCID: PMC3869011
- DOI: 10.1038/ismej.2013.132
Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters
Abstract
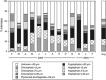
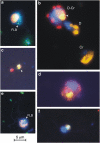
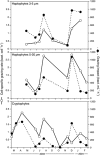
Grazing rate estimates indicate that approximately half of the bacterivory in oligotrophic oceans is due to mixotrophic flagellates (MFs). However, most estimations have considered algae as a single group. Here we aimed at opening the black-box of the phytoflagellates (PFs) <20 μm. Haptophytes, chlorophytes, cryptophytes and pigmented dinoflagellates were identified using fluorescent in situ hybridization or by standard 4',6-diamidino-2-phenylindole staining. Their fluctuations in abundance, cell size, biomass and bacterivory rates were measured through an annual cycle in an oligotrophic coastal system. On average, we were able to assign to these groups: 37% of the total pico-PFs and 65% of the nano-PFs composition. Chlorophytes were mostly picoplanktonic and they never ingested fluorescently labeled bacteria. About 50% of the PF <20 μm biomass was represented by mixotrophic algae. Pigmented dinoflagellates were the least abundant group with little impact on bacterioplankton. Cryptophytes were quantitatively important during the coldest periods and explained about 4% of total bacterivory. Haptophytes were the most important mixotrophic group: (i) they were mostly represented by cells 3-5 μm in size present year-round; (ii) cell-specific grazing rates were comparable to those of other bacterivorous non-photosynthetic organisms, regardless of the in situ nutrient availability conditions; (iii) these organisms could acquire a significant portion of their carbon by ingesting bacteria; and (iv) haptophytes explained on average 40% of the bacterivory exerted by MFs and were responsible for 9-27% of total bacterivory at this site. Our results, when considered alongside the widespread distribution of haptophytes in the ocean, indicate that they have a key role as bacterivores in marine ecosystems.
Figures



References
-
- Alonso-Sáez L, Vázquez-Domínguez E, Cardelús C, Pinhassi J, Sala MM, Lekunberri I, et al. Factors controlling the year-round variability in carbon flux through bacteria in a coastal marine system. Ecosystems. 2008;11:397–409.
-
- Bell EM, Laybourn-Parry J. Mixotrophy in the antarctic phytoflagellate, Pyramimonas gelidicola (Chlorophyta: Prasinophyceae) J Phycol. 2003;39:644–649.
-
- Bird DF, Kalff J. Bacterial grazing by planktonic lake algae. Science. 1986;231:493–495. - PubMed
-
- Caron DA.2001Protistan herbivory and bacterivoryIn: Paul J (ed.)Marine Microbiology, Vol. 30, Methods in Microbiology Academic Press: London; 289–315.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

