Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer
- PMID: 23924790
- PMCID: PMC3779664
- DOI: 10.1097/CJI.0b013e31829fb7a2
Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer
Abstract
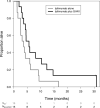
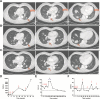
Preclinical reports support the concept of synergy between cancer vaccines and immune checkpoint blockade in nonimmunogenic tumors. In particular, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies have been successfully combined with GM-CSF cell-based vaccines (GVAX). Ipilimumab (anti-CTLA-4) has been tested as a single agent in patients with pancreatic ductal adenocarcinoma (PDA) resulting in a delayed response at a dose of 3 mg/kg. Our study evaluated ipilimumab 10 mg/kg (arm 1) and ipilimumab 10 mg/kg + GVAX (arm 2). A total of 30 patients with previously treated advanced PDA were randomized (1:1). Induction doses were administered every 3 weeks for a total of 4 doses followed by maintenance dosing every 12 weeks. Two patients in arm 1 showed evidence of stable disease (7 and 22 wk) but none demonstrated CA19-9 biochemical responses. In contrast, 3 patients in arm 2 had evidence of prolonged disease stabilization (31, 71, and 81 wk) and 7 patients experienced CA19-9 declines. In 2 of these patients, disease stabilization occurred after an initial period of progression. The median overall survival (OS) (3.6 vs. 5.7 mo, hazards ratio: 0.51, P = 0.072) and 1 year OS (7 vs. 27%) favored arm 2. Similar to prior ipilimumab studies, 20% of patients in each arm had grade 3/4 immune-related adverse events. Among patients with OS > 4.3 months, there was an increase in the peak mesothelin-specific T cells (P = 0.014) and enhancement of the T-cell repertoire (P = 0.031). In conclusion, checkpoint blockade in combination with GVAX has the potential for clinical benefit and should be evaluated in a larger study.
Figures

Comment in
-
Cracking the stone: combination vaccination and CTLA-4 blockade in pancreatic cancer.J Immunother. 2013 Sep;36(7):362-4. doi: 10.1097/CJI.0b013e31829fb7c8. J Immunother. 2013. PMID: 23924787 No abstract available.
References
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. - PubMed
-
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

