Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques
- PMID: 23924801
- PMCID: PMC3851223
- DOI: 10.4161/mabs.25423
Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques
Abstract
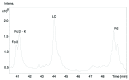
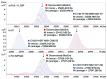
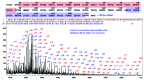
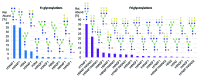
The European Medicines Agency received recently the first marketing authorization application for a biosimilar monoclonal antibody (mAb) and adopted the final guidelines on biosimilar mAbs and Fc-fusion proteins. The agency requires high similarity between biosimilar and reference products for approval. Specifically, the amino acid sequences must be identical. The glycosylation pattern of the antibody is also often considered to be a very important quality attribute due to its strong effect on quality, safety, immunogenicity, pharmacokinetics and potency. Here, we describe a case study of cetuximab, which has been marketed since 2004. Biosimilar versions of the product are now in the pipelines of numerous therapeutic antibody biosimilar developers. We applied a combination of intact, middle-down, middle-up and bottom-up electrospray ionization and matrix assisted laser desorption ionization mass spectrometry techniques to characterize the amino acid sequence and major post-translational modifications of the marketed cetuximab product, with special emphasis on glycosylation. Our results revealed a sequence error in the reported sequence of the light chain in databases and in publications, thus highlighting the potency of mass spectrometry to establish correct antibody sequences. We were also able to achieve a comprehensive identification of cetuximab's glycoforms and glycosylation profile assessment on both Fab and Fc domains. Taken together, the reported approaches and data form a solid framework for the comparability of antibodies and their biosimilar candidates that could be further applied to routine structural assessments of these and other antibody-based products.
Keywords: biosimilar; cetuximab; glycosylation; mass spectrometry; sequence.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
