Elicitation of HIV-1-neutralizing antibodies against the CD4-binding site
- PMID: 23924998
- PMCID: PMC4154626
- DOI: 10.1097/COH.0b013e328363a90e
Elicitation of HIV-1-neutralizing antibodies against the CD4-binding site
Abstract
Purpose of review: The HIV-1 site of binding for the CD4 receptor has long attracted attention as a potential supersite of vulnerability to antibody-mediated neutralization. We review recent findings related to effective CD4-binding site antibodies isolated from HIV-1-infected individuals and discuss implications for immunogen design.
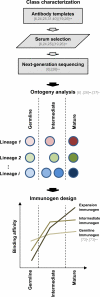
Recent findings: Highly effective CD4-binding site antibodies such as antibody VRC01 have the ability to neutralize over 90% of circulating HIV-1 strains. Sequence and structural analysis of these antibodies from over half a dozen HIV-1-infected donors reveals remarkable similarity in their ontogenies and their modes of recognition, all of which involve mimicry of CD4 receptor by antibody-heavy chain. Meanwhile, other effective CD4-binding site neutralizers such as antibody CH103 have been shown to utilize a different mode of recognition, with next-generation sequencing of both virus and antibody suggesting co-evolution to drive the development of antibody-neutralization breadth.
Summary: The nexus of information concerning the CD4-binding site and its recognition by human antibodies capable of effective neutralization has expanded remarkably in the last few years. Although barriers are substantial, new insights from donor-serum responses, atomic-level structures of antibody-Env complexes, and next-generation sequencing of B-cell transcripts are invigorating vaccine-design efforts to elicit effective CD4-binding site antibodies.
Figures



References
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1884-1888;1998280 - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, et al. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity. 2013;38:176–186. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

