Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice
- PMID: 23925565
- PMCID: PMC3789892
- DOI: 10.1007/s00401-013-1160-3
Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice
Abstract
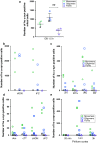
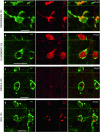
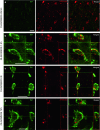
α-Synuclein (α-syn) is a protein prevalent in neural tissue and known to undergo axonal transport. Intracellular α-syn aggregates are a hallmark of Parkinson's disease (PD). Braak and collaborators have suggested that in people who are destined to eventually develop PD, α-syn aggregate pathology progresses following a stereotypic pattern, starting in the olfactory bulb (OB) and the gut. α-Synuclein aggregates are postulated to spread to interconnected brain regions over several years. Thus, propagation of the pathology via neural pathways can potentially explain how α-syn aggregates spread in PD. We have now studied if α-syn can transfer from the OB to other brain structures through neural connections, by injecting different molecular species of human α-syn (monomers, oligomers, fibrils) into the OB of wild-type mice. We found that non-fibrillar human α-syn is taken up very quickly by OB neurons. Within minutes to hours, it is also found in neurons in structures connected to the OB. Conversely, when we injected bovine serum albumin used as a control protein, we found that it does not diffuse beyond the OB, is rarely taken up by OB cells, and does not transfer to other structures. Taken together, our results show that OB cells readily take up α-syn, and that monomeric and oligomeric, but not fibrillar, forms of α-syn are rapidly transferred to interconnected structures within the timeframe we explored. Our results support the idea that α-syn can transfer along neural pathways and thereby contribute to the progression of the α-syn-related pathology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

