Loss of epigenetic Kruppel-like factor 4 histone deacetylase (KLF-4-HDAC)-mediated transcriptional suppression is crucial in increasing vascular endothelial growth factor (VEGF) expression in breast cancer
- PMID: 23926105
- PMCID: PMC3779720
- DOI: 10.1074/jbc.M113.481184
Loss of epigenetic Kruppel-like factor 4 histone deacetylase (KLF-4-HDAC)-mediated transcriptional suppression is crucial in increasing vascular endothelial growth factor (VEGF) expression in breast cancer
Abstract
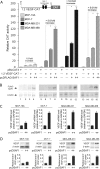
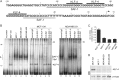
Vascular endothelial growth factor (VEGF) is recognized as an important angiogenic factor that promotes angiogenesis in a series of pathological conditions, including cancer, inflammation, and ischemic disorders. We have recently shown that the inflammatory transcription factor SAF-1 is, at least in part, responsible for the marked increase of VEGF levels in breast cancer. Here, we show that SAF-1-mediated induction of VEGF is repressed by KLF-4 transcription factor. KLF-4 is abundantly present in normal breast epithelial cells, but its level is considerably reduced in breast cancer cells and clinical cancer tissues. In the human VEGF promoter, SAF-1- and KLF-4-binding elements are overlapping, whereas SAF-1 induces and KLF-4 suppresses VEGF expression. Ectopic overexpression of KLF-4 and RNAi-mediated inhibition of endogenous KLF-4 supported the role of KLF-4 as a transcriptional repressor of VEGF and an inhibitor of angiogenesis in breast cancer cells. We show that KLF-4 recruits histone deacetylases (HDACs) -2 and -3 at the VEGF promoter. Chronological ChIP assays demonstrated the occupancy of KLF-4, HDAC2, and HDAC3 in the VEGF promoter in normal MCF-10A cells but not in MDA-MB-231 cancer cells. Co-transfection of KLF-4 and HDAC expression plasmids in breast cancer cells results in synergistic repression of VEGF expression and inhibition of angiogenic potential of these carcinoma cells. Together these results identify a new mechanism of VEGF up-regulation in cancer that involves concomitant loss of KLF-4-HDAC-mediated transcriptional repression and active recruitment of SAF-1-mediated transcriptional activation.
Keywords: Angiogenesis; Breast Cancer; DNA-Protein Interaction; Gene Regulation; Transcription Factors.
Figures





Similar articles
-
Control of VEGF expression in triple-negative breast carcinoma cells by suppression of SAF-1 transcription factor activity.Mol Cancer Res. 2011 Aug;9(8):1030-41. doi: 10.1158/1541-7786.MCR-10-0598. Epub 2011 Jun 10. Mol Cancer Res. 2011. PMID: 21665940
-
Repression of vascular endothelial growth factor expression by the zinc finger transcription factor ZNF24.Cancer Res. 2007 Sep 15;67(18):8736-41. doi: 10.1158/0008-5472.CAN-07-1617. Cancer Res. 2007. PMID: 17875714
-
Induction of Ras by SAF-1/MAZ through a feed-forward loop promotes angiogenesis in breast cancer.Cancer Med. 2015 Feb;4(2):224-34. doi: 10.1002/cam4.362. Epub 2014 Nov 30. Cancer Med. 2015. PMID: 25449683 Free PMC article.
-
Sp/KLF family and tumor angiogenesis in pancreatic cancer.Curr Pharm Des. 2012;18(17):2420-31. doi: 10.2174/13816128112092420. Curr Pharm Des. 2012. PMID: 22372503 Review.
-
Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer.J Cell Physiol. 2001 Aug;188(2):143-60. doi: 10.1002/jcp.1111. J Cell Physiol. 2001. PMID: 11424081 Review.
Cited by
-
LncRNA NORAD Promotes Vascular Endothelial Cell Injury and Atherosclerosis Through Suppressing VEGF Gene Transcription via Enhancing H3K9 Deacetylation by Recruiting HDAC6.Front Cell Dev Biol. 2021 Jul 9;9:701628. doi: 10.3389/fcell.2021.701628. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307380 Free PMC article.
-
Krüppel-like factors in tumors: Key regulators and therapeutic avenues.Front Oncol. 2023 Jan 25;13:1080720. doi: 10.3389/fonc.2023.1080720. eCollection 2023. Front Oncol. 2023. PMID: 36761967 Free PMC article. Review.
-
Identification of placental genes linked to selective intrauterine growth restriction (IUGR) in dichorionic twin pregnancies: gene expression profiling study.Hum Genet. 2019 Jun;138(6):649-659. doi: 10.1007/s00439-019-02016-6. Epub 2019 Apr 30. Hum Genet. 2019. PMID: 31041507 Free PMC article.
-
Epigenetics and environment in breast cancer: New paradigms for anti-cancer therapies.Front Oncol. 2022 Sep 15;12:971288. doi: 10.3389/fonc.2022.971288. eCollection 2022. Front Oncol. 2022. PMID: 36185256 Free PMC article. Review.
-
Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Krüppel-Like Factor 4)-Mediated Histone Modifications.Arterioscler Thromb Vasc Biol. 2019 May;39(5):902-914. doi: 10.1161/ATVBAHA.118.312301. Arterioscler Thromb Vasc Biol. 2019. PMID: 30917677 Free PMC article.
References
-
- Engels K., Fox S. B., Whitehouse R. M., Gatter K. C., Harris A. L. (1997) Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J. Pathol. 181, 207–212 - PubMed
-
- Ferrara N. (1995) The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res. Treat. 36, 127–137 - PubMed
-
- Ferrara N. (2002) Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol. 29, 10–14 - PubMed
-
- Yoshiji H., Gomez D. E., Shibuya M., Thorgeirsson U. P. (1996) Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. 56, 2013–2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

