The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937-2003
- PMID: 23926115
- PMCID: PMC3767966
- DOI: 10.1093/cercor/bht195
The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937-2003
Abstract
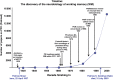
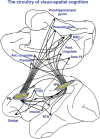
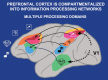
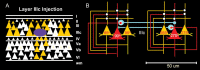
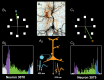
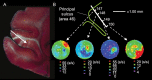
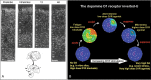
Patricia S. Goldman-Rakic (1937-2003) transformed the study of the prefrontal cortex (PFC) and the neural basis of mental representation, the basic building block of abstract thought. Her pioneering research first identified the dorsolateral PFC (dlPFC) region essential for spatial working memory, and the extensive circuits of spatial cognition. She discovered the cellular basis of working memory, illuminating the dlPFC microcircuitry underlying spatially tuned, persistent firing, whereby precise information can be held "in mind": persistent firing arises from recurrent excitation within glutamatergic pyramidal cell circuits in deep layer III, while tuning arises from GABAergic lateral inhibition. She was the first to discover that dopamine is essential for dlPFC function, particularly through D1 receptor actions. She applied a host of technical approaches, providing a new paradigm for scientific inquiry. Goldman-Rakic's work has allowed the perplexing complexities of mental illness to begun to be understood at the cellular level, including atrophy of the dlPFC microcircuits subserving mental representation. She correctly predicted that impairments in dlPFC working memory activity would contribute to thought disorder, a cardinal symptom of schizophrenia. Ten years following her death, we look back to see how she inspired an entire field, fundamentally changing our view of cognition and cognitive disorders.
Keywords: dopamine; mental representation; prefrontal cortex; schizophrenia; working memory.
Figures


References
-
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban NB, Narendran R, Hwang DR, Laruelle M, Slifstein M. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2012;26:794–805. - PubMed
-
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. - PubMed
-
- Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. - PubMed
-
- Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. - PubMed
Publication types
MeSH terms
Personal name as subject
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

