Autonomic dysreflexia causes chronic immune suppression after spinal cord injury
- PMID: 23926252
- PMCID: PMC3735880
- DOI: 10.1523/JNEUROSCI.1974-13.2013
Autonomic dysreflexia causes chronic immune suppression after spinal cord injury
Abstract
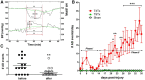
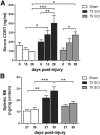
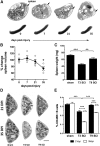
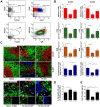
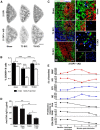
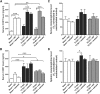
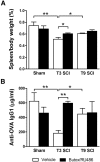
Autonomic dysreflexia (AD), a potentially dangerous complication of high-level spinal cord injury (SCI) characterized by exaggerated activation of spinal autonomic (sympathetic) reflexes, can cause pulmonary embolism, stroke, and, in severe cases, death. People with high-level SCI also are immune compromised, rendering them more susceptible to infectious morbidity and mortality. The mechanisms underlying postinjury immune suppression are not known. Data presented herein indicate that AD causes immune suppression. Using in vivo telemetry, we show that AD develops spontaneously in SCI mice with the frequency of dysreflexic episodes increasing as a function of time postinjury. As the frequency of AD increases, there is a corresponding increase in splenic leucopenia and immune suppression. Experimental activation of spinal sympathetic reflexes in SCI mice (e.g., via colorectal distension) elicits AD and exacerbates immune suppression via a mechanism that involves aberrant accumulation of norepinephrine and glucocorticoids. Reversal of postinjury immune suppression in SCI mice can be achieved by pharmacological inhibition of receptors for norepinephrine and glucocorticoids during the onset and progression of AD. In a human subject with C5 SCI, stimulating the micturition reflex caused AD with exaggerated catecholamine release and impaired immune function, thus confirming the relevance of the mouse data. These data implicate AD as a cause of secondary immune deficiency after SCI and reveal novel therapeutic targets for overcoming infectious complications that arise due to deficits in immune function.
Figures


References
-
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
