Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport
- PMID: 23926272
- PMCID: PMC3735891
- DOI: 10.1523/JNEUROSCI.0935-13.2013
Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport
Abstract
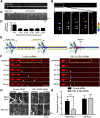
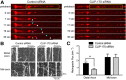
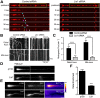
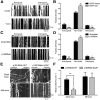
Long-range retrograde axonal transport in neurons is driven exclusively by the microtubule motor cytoplasmic dynein. The efficient initiation of dynein-mediated transport from the distal axon is critical for normal neuronal function, and neurodegenerative disease-associated mutations have been shown to specifically disrupt this process. Here, we examine the role of dynamic microtubules and microtubule plus-end binding proteins (+TIPs) in the initiation of dynein-mediated retrograde axonal transport using live-cell imaging of cargo motility in primary mouse dorsal root ganglion neurons. We show that end-binding (EB)-positive dynamic microtubules are enriched in the distal axon. The +TIPs EB1, EB3, and cytoplasmic linker protein-170 (CLIP-170) interact with these dynamic microtubules, recruiting the dynein activator dynactin in an ordered pathway, leading to the initiation of retrograde transport by the motor dynein. Once transport has initiated, however, neither the EBs nor CLIP-170 are required to maintain transport flux along the mid-axon. In contrast, the +TIP Lis1 activates transport through a distinct mechanism and is required to maintain processive organelle transport along both the distal and mid-axon. Further, we show that the EB/CLIP-170/dynactin-dependent mechanism is required for the efficient initiation of transport from the distal axon for multiple distinct cargos, including mitochondria, Rab5-positive early endosomes, late endosomes/lysosomes, and TrkA-, TrkB-, and APP-positive organelles. Our observations indicate that there is an essential role for +TIPs in the regulation of retrograde transport initiation in the neuron.
Figures


References
-
- Akhmanova A, Mausset-Bonnefont AL, van Cappellen W, Keijzer N, Hoogenraad CC, Stepanova T, Drabek K, van der Wees J, Mommaas M, Onderwater J, van der Meulen H, Tanenbaum ME, Medema RH, Hoogerbrugge J, Vreeburg J, Uringa EJ, Grootegoed JA, Grosveld F, Galjart N. The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes and development. 2005;19:2501–2515. doi: 10.1101/gad.344505. - DOI - PMC - PubMed
-
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
