Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia
- PMID: 23926302
- PMCID: PMC3795464
- DOI: 10.1182/blood-2013-06-510461
Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia
Abstract
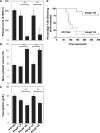
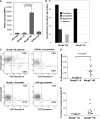
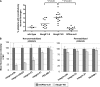
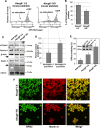
Sitosterolemia is a rare, autosomal recessive disease caused by mutations in the adenosine triphosphate-binding cassette transporter genes ABCG5 or ABCG8 that result in accumulation of xenosterols in the body. Clinical manifestations include tendon xanthomas, premature coronary artery disease, hemolytic anemia, macrothrombocytopenia, and bleeding. Although the effect of sterol accumulation on the predisposition for atherosclerosis is evident, how xenosterol accumulation leads to defects in platelet physiology is unknown. Sitosterolemia induced in Abcg5- and Abcg8-deficient mice fed a high plant sterol diet resulted in accumulation of free sterols in platelet plasma membranes, leading to hyperactivatable platelets characterized by constitutive binding of fibrinogen to its αIIbβ3 integrin receptor, internalization of the αIIbβ3 complex, generation of platelet-derived microparticles, and changes in the quantity and subcellular localization of filamin. The latter was associated with macrothrombocytopenia, shedding of GPIbα, impaired platelet adhesion to von Willebrand factor, and inability to form stable thrombi. Plasma levels of soluble GPIbα were strongly correlated with plasma sitosterol levels in samples from human sitosterolemic patients, implicating a similar mechanism of sterol-induced platelet passivation in the human disease. Intercalation of plant sterols into the plasma membrane therefore results in dysregulation of multiple platelet activation pathways, leading to macrothrombocytopenia and bleeding.
Figures
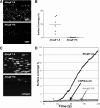
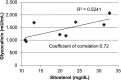
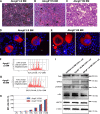
Comment in
-
Sitosterolemia: platelets on high-sterol diet.Blood. 2013 Oct 10;122(15):2534-5. doi: 10.1182/blood-2013-08-521153. Blood. 2013. PMID: 24113795 Free PMC article.
Similar articles
-
Specific macrothrombocytopenia/hemolytic anemia associated with sitosterolemia.Am J Hematol. 2014 Mar;89(3):320-4. doi: 10.1002/ajh.23619. Am J Hematol. 2014. PMID: 24166850
-
Genetic basis and hematologic manifestations of sitosterolemia in a group of Turkish patients.J Clin Lipidol. 2021 Sep-Oct;15(5):690-698. doi: 10.1016/j.jacl.2021.07.001. Epub 2021 Jul 10. J Clin Lipidol. 2021. PMID: 34304999
-
Two novel variants of the ABCG5 gene cause xanthelasmas and macrothrombocytopenia: a brief review of hematologic abnormalities of sitosterolemia.J Thromb Haemost. 2017 Sep;15(9):1859-1866. doi: 10.1111/jth.13777. Epub 2017 Aug 5. J Thromb Haemost. 2017. PMID: 28696550 Review.
-
Plant Sterols, Stanols, and Sitosterolemia.J AOAC Int. 2015 May-Jun;98(3):716-723. doi: 10.5740/jaoacint.SGEAjagbe. Epub 2015 May 4. J AOAC Int. 2015. PMID: 25941971 Free PMC article. Review.
-
Timely diagnosis of sitosterolemia by next generation sequencing in two children with severe hypercholesterolemia.Atherosclerosis. 2017 Jul;262:71-77. doi: 10.1016/j.atherosclerosis.2017.05.002. Epub 2017 May 4. Atherosclerosis. 2017. PMID: 28521186
Cited by
-
Sitosterolemia: a review and update of pathophysiology, clinical spectrum, diagnosis, and management.Ann Pediatr Endocrinol Metab. 2016 Mar;21(1):7-14. doi: 10.6065/apem.2016.21.1.7. Epub 2016 Mar 31. Ann Pediatr Endocrinol Metab. 2016. PMID: 27104173 Free PMC article. Review.
-
Genetics of familial hypercholesterolemia.Curr Atheroscler Rep. 2015 Apr;17(4):491. doi: 10.1007/s11883-015-0491-z. Curr Atheroscler Rep. 2015. PMID: 25712136 Review.
-
Sitosterolemia: platelets on high-sterol diet.Blood. 2013 Oct 10;122(15):2534-5. doi: 10.1182/blood-2013-08-521153. Blood. 2013. PMID: 24113795 Free PMC article.
-
Clinical characteristics of sitosterolemic children with xanthomas as the first manifestation.Lipids Health Dis. 2022 Oct 13;21(1):100. doi: 10.1186/s12944-022-01710-1. Lipids Health Dis. 2022. PMID: 36229885 Free PMC article.
-
Sitosterolemia: Four Cases of an Uncommon Cause of Hemolytic Anemia (Mediterranean Stomatocytosis with Macrothrombocytopenia).Indian J Hematol Blood Transfus. 2021 Jan;37(1):157-161. doi: 10.1007/s12288-020-01346-0. Epub 2020 Oct 22. Indian J Hematol Blood Transfus. 2021. PMID: 33707850 Free PMC article.
References
-
- Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res. 1999;40(2):302–308. - PubMed
-
- Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18(8):652–662. - PubMed
-
- Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290(5497):1771–1775. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

