The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo
- PMID: 23926321
- PMCID: PMC4020118
- DOI: 10.4049/jimmunol.1300748
The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo
Abstract
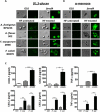
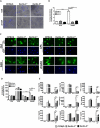
Aspergillus and Fusarium species are important causes of fungal infections worldwide. Airborne spores (conidia) of these filamentous fungi express a surface protein that confers hydrophobicity (hydrophobin) and covers cell wall components that would otherwise induce a host immune cell response. Using a mutant Aspergillus fumigatus strain (ΔrodA) that does not express the RodA hydrophobin, and Aspergillus and Fusarium conidia from clinical isolates that were treated with hydrofluoric acid (which removes the A. fumigatus RodA protein), we observed increased surface exposure of β1,3-glucan and α-mannose on Aspergillus and Fusarium conidia. We also found that ΔrodA and hydrofluoric acid-treated conidia stimulate significantly higher NF-κB p65 nuclear translocation and cytokine production by macrophages from C57BL/6, but not from Dectin-1(-/-) or Dectin-2(-/-) mice. Using a murine model of A. fumigatus corneal infection, we showed that ΔrodA conidia induced significantly higher cytokine production, neutrophil infiltration, and more rapid fungal clearance from C57BL/6 corneas compared with the parent G10 strain, which was dependent on Dectin-1 and Dectin-2. Together, these findings identify the hydrophobin RodA as a virulence factor that masks Dectin-1 and Dectin-2 recognition of conidia, resulting in impaired neutrophil recruitment to the cornea and increased fungal survival and clinical disease.
Figures


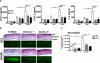

References
-
- Oliveira M, Ribeiro H, Delgado JL, Abreu I. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. Int J Biometeorol. 2009;53:61–73. - PubMed
-
- Mahieu LM, De Dooy JJ, Van Laer FA, Jansens H, Ieven MM. A prospective study on factors influencing aspergillus spore load in the air during renovation works in a neonatal intensive care unit. J Hosp Infect. 2000;45:191–197. - PubMed
-
- Latge JP. Tasting the fungal cell wall. Cell Microbiol. 2010;12:863–872. - PubMed
-
- Linder MB, Szilvay GR, Nakari-Setala T, Penttila ME. Hydrophobins: the protein-amphiphiles of filamentous fungi. Fems Microbiology Reviews. 2005;29:877–896. - PubMed
-
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

