Quetiapine versus haloperidol in the treatment of delirium: a double-blind, randomized, controlled trial
- PMID: 23926422
- PMCID: PMC3728270
- DOI: 10.2147/DDDT.S45575
Quetiapine versus haloperidol in the treatment of delirium: a double-blind, randomized, controlled trial
Abstract
Background: Atypical antipsychotic drugs may have low propensity to induce extrapyramidal side effects in delirious patients. This study aimed to compare the efficacy and tolerability between quetiapine and haloperidol in controlling delirious behavior.
Methods: A 7-day prospective, double-blind, randomized controlled trial was conducted from June 2009 to April 2011 in medically ill patients with delirium. Measures used for daily assessment included the Delirium Rating Scale-revised-98 (DRS-R-98) and total sleep time. The Clinical Global Impression, Improvement (CGI-I) and the Modified (nine-item) Simpson- Angus Scale were applied daily. The primary outcome was the DRS-R-98 severity scores. The data were analyzed on an intention-to-treat basis.
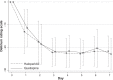
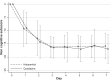
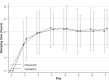
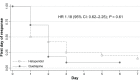
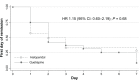
Results: Fifty-two subjects (35 males and 17 females) were randomized to receive 25-100 mg/day of quetiapine (n = 24) or 0.5-2.0 mg/day of haloperidol (n = 28). Mean (standard deviation) doses of quetiapine and haloperidol were 67.6 (9.7) and 0.8 (0.3) mg/day, respectively. Over the trial period, means (standard deviation) of the DRS-R-98 severity scores were not significantly different between the quetiapine and haloperidol groups (-22.9 [6.9] versus -21.7 [6.7]; P = 0.59). The DRS-R-98 noncognitive and cognitive subscale scores were not significantly different. At end point, the response and remission rates, the total sleep time, and the Modified (nine-item) Simpson-Angus scores were also not significantly different between groups. Hypersomnia was common in the quetiapine-treated patients (33.3%), but not significantly higher than that in the haloperidol-treated group (21.4%).
Limitations: Patients were excluded if they were not able to take oral medications, and the sample size was small.
Conclusion: Low-dose quetiapine and haloperidol may be equally effective and safe for controlling delirium symptoms.
Clinical trials registration number: clinicaltrials.gov NCT00954603.
Keywords: delirium; extrapyramidal symptoms; haloperidol; quetiapine.
Figures

References
-
- Maneeton B, Khemawichanurat W, Maneeton N. Consultation-liaison psychiatry in Maharaj Nakorn Chiang Mai Hospital. ASEAN Journal of Psychiatry. 2007;8(2):124–130.
-
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. - PubMed
-
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics. 2009;50(3):234–238. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

