Transjecting growth hormone: continuous nightmare or controlled nuisance? Evaluation of a new needle-free device
- PMID: 23926423
- PMCID: PMC3728268
- DOI: 10.2147/PPA.S46990
Transjecting growth hormone: continuous nightmare or controlled nuisance? Evaluation of a new needle-free device
Abstract
Background: Administering growth-hormone therapy (GHT) is a long-term treatment, associated with avoidance and phobic behaviors in the children involved. The current study examined GHT users' perceptions of a new needle-free device (ZomaJet Vision X [10 mg/mL]) with a lower injection volume compared to the traditional device.
Methods: A total of 73 persons participated (mean age ± standard deviation, 10.10 ± 3.60 years) in a longitudinal design. Users' views were studied 4 weeks after having applied both the old and the new device for a period of at least 4 weeks. Satisfaction, ease and frequency of restitution, local sensations, bruises during administering GHT, affective response to local sensations, and subject preference were assessed on the basis of the users' responses.
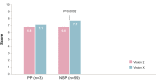
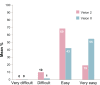
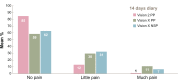
Results: Subjects' satisfaction with the new device was equal compared with the previous device for the total group of 73 children. However, the subgroup of 59 children who proved tolerant to meta-cresol (new preservative for Vision X only) reported a significantly higher satisfaction rating with the new device compared to the old device (7.7 vs 6.6, P=0.0002). Vision X was evaluated as better on ease and frequency of restitution and the number of bruises. Pain sensations did not differ meaningfully between the two devices. The new device was favored over the previous one in a majority of respondents. Vision X allows easy reconstitution of the solution, which was reflected in the percentage of young children able to prepare transjections themselves being more than doubled, illustrating the greater sense of empowerment in these users. Self-reported adherence to the therapy was good (less than 10% of injections missed) with both devices.
Conclusion: The new device ZomaJet Vision X appears to be evaluated more positively than the previous version on criteria that refect users' preferences.
Keywords: children; growth-hormone therapy; invasive medical procedures; patient perception; transjection.
Figures

References
-
- Andrews GJ. ‘I had to go to the hospital and it was freaking me out’: needle phobic encounter space. Health Place. 2011;17:875–884. - PubMed
-
- Duff AJ, Gaskell SL, Jacobs K, Houghton JM. Management of distressing procedures in children and young people: time to adhere to the guidelines. Arch Dis Child. 2012;97:1–4. - PubMed
-
- National Institute for Health and Care Excellence Human growth hormone (somatropin) for the treatment of growth failure in children (review) (TA188) 2010Available from: http://guidance.nice.org.uk/TA188Accessed June 5, 2013
-
- Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14:143–154. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

