The essential nature of iron usage and regulation
- PMID: 23928078
- PMCID: PMC3928970
- DOI: 10.1016/j.cub.2013.05.033
The essential nature of iron usage and regulation
Erratum in
- Curr Biol. 2013 Nov 18;23(22):2325
-
The essential nature of iron usage and regulation.Curr Biol. 2013 Nov 18;23(22):2325. doi: 10.1016/j.cub.2013.10.059. Epub 2013 Nov 18. Curr Biol. 2013. PMID: 30001585 No abstract available.
Abstract
The facile ability of iron to gain and lose electrons has made iron an important participant in a wide variety of biochemical reactions. Binding of ligands to iron modifies its redox potential, thereby permitting iron to transfer electrons with greater or lesser facility. The ability to transfer electrons, coupled with its abundance, as iron is the fourth most abundant mineral in the earth's crust, have contributed to iron being an element required by almost all species in the six kingdoms of life. Iron became an essential element for both Eubacteria and Archeabacteria in the early oxygen-free stages of the earth's evolution. With the advent of an oxygen-rich environment, the redox properties of iron made it extremely useful, as much of iron utilization in eukaryotes is focused on oxygen metabolism, either as an oxygen carrier or as an electron carrier that can facilitate oxygen-based chemistry.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
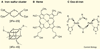
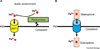

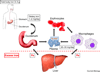

References
-
- Hantke K. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 2001;4:172–177. - PubMed
-
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. - PubMed
-
- Kaplan CD, Kaplan J. Iron acquisition and transcriptional regulation. Chem. Rev. 2009;109:4536–4552. - PubMed
-
- Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012;63:131–152. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

