Phospholipid-modified polyethylenimine-based nanopreparations for siRNA-mediated gene silencing: implications for transfection and the role of lipid components
- PMID: 23928214
- PMCID: PMC3916950
- DOI: 10.1016/j.nano.2013.07.016
Phospholipid-modified polyethylenimine-based nanopreparations for siRNA-mediated gene silencing: implications for transfection and the role of lipid components
Abstract
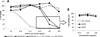
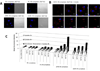
The clinical application of gene silencing mediated by small interfering RNA (siRNA) has been limited by the lack of efficient and safe carriers. Phospholipid modification of low molecular weight polyethylenimine (PEI 1.8 kDa) dramatically increased its gene down-regulation capacity while keeping cytotoxicity levels low. The silencing efficacy was highly dependent on the nature of the lipid grafted to PEI and the polymer/siRNA ratio employed. Phosphoethanolamine (DOPE and DPPE) and phosphocholine (PC) conjugation did not change the physicochemical properties and siRNA binding capacity of PEI complexes but had a large impact on their transfection and ability to down-regulate Green Fluorescent Protein (GFP) expression (60%, 30% and 5% decrease of GFP expression respectively). We found that the micelle-forming structure of DOPE and DPPE-PEI dramatically changed PEI's interaction with cell membranes and played a key role in promoting PEI 1.8 kDa transfection, completely ineffective in the absence of the lipid modification.
From the clinical editor: While siRNA-based gene silencing methods could have numerous clinical applications, efficient delivery remains a major challenge. This team reports that DOPE-PEI and DPPE-PEI based micelle-forming nanostructures may be able to provide an efficient vector for siRNA transfection.
Keywords: Lipid conjugation; Micelle; Phospholipid; Polyethylenimine; RNA interference; siRNA delivery.
© 2014.
Figures


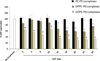

References
-
- de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. International journal of pharmaceutics. 2007;331:167–175. - PubMed
-
- van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:1393–1397. - PubMed
-
- de Martimprey H, Vauthier C, Malvy C, Couvreur P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2009;71:490–504. - PubMed
-
- David S, Pitard B, Benoit JP, Passirani C. Non-viral nanosystems for systemic siRNA delivery. Pharmacological research : the official journal of the Italian Pharmacological Society. 2010;62:100–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

