YidC protein, a molecular chaperone for LacY protein folding via the SecYEG protein machinery
- PMID: 23928306
- PMCID: PMC3784728
- DOI: 10.1074/jbc.M113.491613
YidC protein, a molecular chaperone for LacY protein folding via the SecYEG protein machinery
Abstract
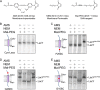
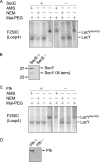
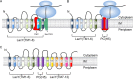
To understand how YidC and SecYEG function together in membrane protein topogenesis, insertion and folding of the lactose permease of Escherichia coli (LacY), a 12-transmembrane helix protein LacY that catalyzes symport of a galactoside and an H(+), was studied. Although both the SecYEG machinery and signal recognition particle are required for insertion of LacY into the membrane, YidC is not required for translocation of the six periplasmic loops in LacY. Rather, YidC acts as a chaperone, facilitating LacY folding. Upon YidC depletion, the conformation of LacY is perturbed, as judged by monoclonal antibody binding studies and by in vivo cross-linking between introduced Cys pairs. Disulfide cross-linking also demonstrates that YidC interacts with multiple transmembrane segments of LacY during membrane biogenesis. Moreover, YidC is strictly required for insertion of M13 procoat protein fused into the middle cytoplasmic loop of LacY. In contrast, the loops preceding and following the inserted procoat domain are dependent on SecYEG for insertion. These studies demonstrate close cooperation between the two complexes in membrane biogenesis and that YidC functions primarily as a foldase for LacY.
Keywords: Chaperone; Chaperonin; LacY; Membrane Biogenesis; Membrane Enzymes; Membrane Insertion; Membrane Proteins; Protein Folding; YidC.
Figures






Similar articles
-
YidC assists the stepwise and stochastic folding of membrane proteins.Nat Chem Biol. 2016 Nov;12(11):911-917. doi: 10.1038/nchembio.2169. Epub 2016 Sep 5. Nat Chem Biol. 2016. PMID: 27595331 Free PMC article.
-
Role of YidC in folding of polytopic membrane proteins.J Cell Biol. 2004 Apr;165(1):53-62. doi: 10.1083/jcb.200402067. Epub 2004 Apr 5. J Cell Biol. 2004. PMID: 15067017 Free PMC article.
-
Insertion and folding pathways of single membrane proteins guided by translocases and insertases.Sci Adv. 2019 Jan 30;5(1):eaau6824. doi: 10.1126/sciadv.aau6824. eCollection 2019 Jan. Sci Adv. 2019. PMID: 30801000 Free PMC article.
-
Mechanisms of YidC-mediated insertion and assembly of multimeric membrane protein complexes.J Biol Chem. 2008 Nov 14;283(46):31269-73. doi: 10.1074/jbc.R800029200. Epub 2008 Jul 25. J Biol Chem. 2008. PMID: 18658156 Review.
-
YidC as a potential antibiotic target.Biochim Biophys Acta Mol Cell Res. 2023 Feb;1870(2):119403. doi: 10.1016/j.bbamcr.2022.119403. Epub 2022 Nov 23. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36427551 Review.
Cited by
-
YidC assists the stepwise and stochastic folding of membrane proteins.Nat Chem Biol. 2016 Nov;12(11):911-917. doi: 10.1038/nchembio.2169. Epub 2016 Sep 5. Nat Chem Biol. 2016. PMID: 27595331 Free PMC article.
-
The mechanisms of integral membrane protein biogenesis.Nat Rev Mol Cell Biol. 2022 Feb;23(2):107-124. doi: 10.1038/s41580-021-00413-2. Epub 2021 Sep 23. Nat Rev Mol Cell Biol. 2022. PMID: 34556847 Free PMC article. Review.
-
Substrate-driven assembly of a translocon for multipass membrane proteins.Nature. 2022 Nov;611(7934):167-172. doi: 10.1038/s41586-022-05330-8. Epub 2022 Oct 19. Nature. 2022. PMID: 36261522 Free PMC article.
-
Unfolding of a ClC chloride transporter retains memory of its evolutionary history.Nat Chem Biol. 2018 May;14(5):489-496. doi: 10.1038/s41589-018-0025-4. Epub 2018 Mar 26. Nat Chem Biol. 2018. PMID: 29581582 Free PMC article.
-
Applications of Single-Molecule Methods to Membrane Protein Folding Studies.J Mol Biol. 2018 Feb 16;430(4):424-437. doi: 10.1016/j.jmb.2017.05.021. Epub 2017 May 23. J Mol Biol. 2018. PMID: 28549924 Free PMC article. Review.
References
-
- Xie K., Dalbey R. E. (2008) Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat. Rev. Microbiol. 6, 234–244 - PubMed
-
- Driessen A. J., Nouwen N. (2008) Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77, 643–667 - PubMed
-
- Dalbey R. E., Wang P., Kuhn A. (2011) Assembly of bacterial inner membrane proteins. Annu. Rev. Biochem. 80, 161–187 - PubMed
-
- Luirink J., Yu Z., Wagner S., de Gier J. W. (2012) Biogenesis of inner membrane proteins in Escherichia coli. Biochim. Biophys. Acta 1817, 965–976 - PubMed
-
- Stephenson K. (2005) Sec-dependent protein translocation across biological membranes. Evolutionary conservation of an essential protein transport pathway (review). Mol. Membr. Biol. 22, 17–28 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

