Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death
- PMID: 23929941
- PMCID: PMC3820268
- DOI: 10.1242/dmm.010447
Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death
Abstract
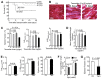
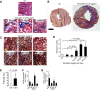
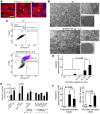
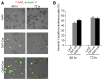
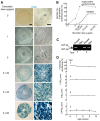
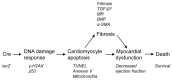
Numerous mouse models have utilized Cre-loxP technology to modify gene expression. Adverse effects of Cre recombinase activity have been reported, including in the heart. However, the mechanisms associated with cardiac Cre toxicity are largely unknown. Here, we show that expression of Cre in cardiomyocytes induces a DNA damage response, resulting in cardiomyocyte apoptosis, cardiac fibrosis and cardiac dysfunction. In an effort to increase the recombination efficiency of a widely used tamoxifen-sensitive Cre transgene under control of the α-myosin-heavy-chain promoter (αMHC-MerCreMer), we observed myocardial dysfunction and decreased survival, which were dependent on the dose of tamoxifen injected. After excluding a Cre-independent contribution by tamoxifen, we found that Cre induced myocardial fibrosis, activation of pro-fibrotic genes and cardiomyocyte apoptosis. Examination of the molecular mechanisms showed activation of DNA damage response signaling and p53 stabilization in the absence of loxP sites, suggesting that Cre induced illegitimate DNA breaks. Cardiomyocyte apoptosis was also induced by expressing Cre using adenoviral transduction, indicating that the effect was not dependent on genomic integration of the transgene. Cre-mediated homologous recombination at loxP sites was dose-dependent and had a ceiling effect at ∼80% of cardiomyocytes showing recombination. By titrating the amount of tamoxifen to maximize recombination while minimizing animal lethality, we determined that 30 μg tamoxifen/g body weight/day injected on three consecutive days is the optimal condition for the αMHC-MerCreMer system to induce recombination in the Rosa26-lacZ strain. Our results further highlight the importance of experimental design, including the use of appropriate genetic controls for Cre expression.
Figures
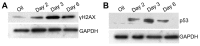
References
-
- Bae S., Yalamarti B., Kang P. M. (2008). Role of caspase-independent apoptosis in cardiovascular diseases. Front. Biosci. 13, 2495–503 - PubMed
-
- Bahi N., Zhang J., Llovera M., Ballester M., Comella J. X., Sanchis D. (2006). Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J. Biol. Chem. 281, 22943–22952 - PubMed
-
- Bersell K., Arab S., Haring B., Kühn B. (2009). Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 - PubMed
-
- Buerger A., Rozhitskaya O., Sherwood M. C., Dorfman A. L., Bisping E., Abel E. D., Pu W. T., Izumo S., Jay P. Y. (2006). Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J. Card. Fail. 12, 392–398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

