A Microfluidic Passive Pumping Coulter Counter
- PMID: 23930109
- PMCID: PMC3735229
- DOI: 10.1007/s10404-010-0609-0
A Microfluidic Passive Pumping Coulter Counter
Abstract
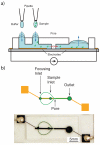
A microfluidic device using on-chip passive pumping was characterized for use as a particle counter. Flow occurred due to a Young-Laplace pressure gradient between two 1.2 mm diameter inlets and a 4 mm diameter reservoir when 0.5μ L fluid droplets were applied to the inlets using a micropipette. Polystyrene particles (10μm diameter) were enumerated using the resistive pulse technique. Particle counts using passive pumping were within 13% of counts from a device using syringe pumping. All pumping methods produced particle counts that were within 16% of those obtained with a hemocytometer. The effect of intermediate wash steps on particle counts within the passive pumping device was determined. Zero, one, or two wash droplets were loaded after the first of two sample droplets. No statistical difference was detected in the mean particle counts among the loading patterns (p > 0.05). Hydrodynamic focusing using passive pumping was also demonstrated.
Keywords: Colloid; Enumeration; Microfluidics; Resistive Pulse.
Figures



References
-
- Arndt S, Seebach J, Psathaki K, Galla H, Wegener J. Bioelectrical impedance assay to monitor changes in cell shape during apoptosis. Biosens Bioelectron. 2004;19(6):583–594. - PubMed
-
- Ateya D, Sachs F, Gottlieb P, Besch S, Hua S. Volume cytometry: Microfluidic sensor for high-throughput screening in real time. Analytical Chemistry. 2005;77(5):1290–1294. - PubMed
-
- Benazzi G, Holmes D, Sun T, Mowlem MC, Morgan H. Discrimination and analysis of phytoplankton using a microfluidic cytometer. IET Nanobiotechnol. 2007;1(6):94–101. - PubMed
-
- Berthier E, Beebe DJ. Flow rate analysis of a surface tension driven passive micropump. Lab Chip. 2007;7(11):1475–1478. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
