Advancement of Imidazo[1,2- a]pyridines with Improved Pharmacokinetics and Nanomolar Activity Against Mycobacterium tuberculosis
- PMID: 23930153
- PMCID: PMC3733398
- DOI: 10.1021/ml400088y
Advancement of Imidazo[1,2- a]pyridines with Improved Pharmacokinetics and Nanomolar Activity Against Mycobacterium tuberculosis
Abstract
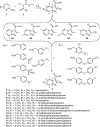
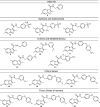
A set of fourteen imidazo[1,2-a]pyridine-3-carboxamides was synthesized and screened against Mycobacterium tuberculosis H37Rv. The minimum inhibitory concentrations of twelve of these agents were ≤ 1 μM against replicating bacteria and five compounds (9, 12, 16, 17 and 18) had MIC values ≤ 0.006 μM. Compounds 13 and 18 were screened against a panel of MDR and XDR drug resistant clinical Mtb strains with the potency of 18 surpassing that of clinical candidate PA-824 by nearly 10 fold. The in vivo pharmacokinetics of compounds 13 and 18 were evaluated in male mice by oral (PO) and intravenous (IV) routes. These results indicate that readily synthesized imidazo[1,2-a]pyridine-3-carboxamides are an exciting new class of potent, selective anti-TB agents that merit additional development opportunities.
Keywords: MDR-TB; Mycobacterium tuberculosis; XDR-TB; imidazo[1,2-a]pyridine-3-carboxamides; pharmacokinetics.
Figures
References
-
- WHO. Global Tuberculosis Control WHO Report 2011; WHO/HTM/TB/2011.16, 2011.
-
- Moraski G. C.; Markley L. D.; Chang M.; Cho S.; Franzblau S. G.; Hwang C. H.; Boshoff H.; Miller M. J. Generation and exploration of new classes of antitubercular agents: The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds. Bioorg. Med. Chem. 2012, 7, 2214–2220. - PMC - PubMed
-
- Mak P. M.; Rao S. P. S.; Tan M.-P.; Lin X.; Chyba J.; Tay J.; Ng S.-H.; Tan B.-H.; Cherian J.; Duraiswamy J.; Bifani P.; Vim V.; Lee B.-H.; Ma N.-L.; Beer D.; Thayalan P.; Kuhen K.; Chatterjee A.; Supek F.; Glynne R.; Zheng J.; Boshoff H. I.; Barr C. E.; Dick T.; Pethe K.; Camacho L. R. A High-Throughput Screen To Identify Inhibitors of ATP Homeostasis in Non-replicating Mycobacterium tuberculosis. ACS Chem. Biol. 2012, 7, 1190–1197. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

