DNA strands with alternating incorporations of LNA and 2'- O-(pyren-1-yl)methyluridine: SNP-discriminating RNA detection probes
- PMID: 23930202
- PMCID: PMC3733389
- DOI: 10.1039/C3SC50726B
DNA strands with alternating incorporations of LNA and 2'- O-(pyren-1-yl)methyluridine: SNP-discriminating RNA detection probes
Abstract
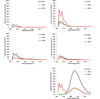
Detection of nucleic acids using fluorophore-modified oligonucleotides forms the basis of many important applications in molecular biology, genetics and medical diagnostics. Here we demonstrate that DNA strands with central segments of alternating locked nucleic acid (LNA) and 2'-O-(pyren-1-yl)methyluridine monomers display very large and highly mismatch-sensitive increases in fluorescence emission upon RNA hybridization, whereas corresponding "LNA-free" controls do not. Absorbance spectra strongly suggest that LNA-induced conformational tuning of flanking 2'-O-(pyren-1-yl)methyluridine monomers places the reporter group in the minor groove upon RNA binding, whereby pyrene-nucleobase interactions leading to quenching of fluorescence are minimized. Accordingly, these easy-to-synthesize probes are promising SNP-discriminating RNA detection probes.
Figures


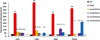

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

