Phase II study of TAS-106 in patients with platinum-failure recurrent or metastatic head and neck cancer and nasopharyngeal cancer
- PMID: 23930212
- PMCID: PMC3699847
- DOI: 10.1002/cam4.79
Phase II study of TAS-106 in patients with platinum-failure recurrent or metastatic head and neck cancer and nasopharyngeal cancer
Abstract
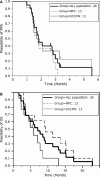
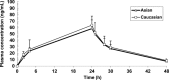
TAS-106, a RNA polymerase inhibitor, was studied in solid tumors with potential clinical benefit and reasonable tolerability. We conducted a multicenter, international phase II trial of TAS-106 in salvage metastatic or recurrent head and neck squamous cell cancer (HNSCC) and nasopharyngeal cancer (NPC) patients. TAS-106 monotherapy was given at 6.5 mg/m(2) over 24-h continuous infusion every 3 weeks. Translational studies for blood and tissue were included. Twenty-seven enrolled patients experienced the most common drug-related adverse events of neutropenia, fatigue, non-neutropenic fever, injection site reaction, and skin rash/dermatitis. The greater than or equal to grade 3 adverse events included neutropenia (14.8%), febrile neutropenia (7.4%), pneumonia (7.4%), and peripheral neuropathy (3.7%). The overall response rate was 0% in both subgroups; five HNSCC patients had stable disease (median duration 99 days) and four NPC patients had stable disease (median duration of 92.5 days). Median progression-free survival (PFS) for HNSCC patients was 52 days (95% CI 43.0-99.0 days) and 48 days (95% CI 41.0-83.0 days) for NPC. Median overall survival (OS) for HNSCC patients was 175 days (95% CI 92.0-234.0 days) and 280 days (95% CI 107.0-462.0 days) for NPC. The TAS-106 plasma levels were equivalent between Asian and Caucasian patients. There was no significant correlation of tumor UCK2 protein expression levels to TAS-106 efficacy. TAS-106 was reasonably tolerated in patients with platinum-failure HNSCC and NPC. The administration schedule of 24-h continuous infusion prevented neurologic toxicity, but had myelosuppression as its main toxicity. There was no anti-tumor efficacy seen with TAS-106 monotherapy. Future studies will focus on TAS-106 combinations and mechanisms of drug resistance.
Keywords: Head and neck squamous cell carcinoma; TAS-106; nasopharyngeal cancer.
Figures
Similar articles
-
Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial.Lancet Oncol. 2015 May;16(5):583-94. doi: 10.1016/S1470-2045(15)70124-5. Epub 2015 Apr 16. Lancet Oncol. 2015. PMID: 25892145 Clinical Trial.
-
Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma.J Clin Oncol. 2007 Aug 20;25(24):3766-73. doi: 10.1200/JCO.2006.10.2871. J Clin Oncol. 2007. PMID: 17704426 Clinical Trial.
-
Randomized Phase II Study of Cabazitaxel Versus Methotrexate in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Previously Treated With Platinum-Based Therapy.Oncologist. 2016 Dec;21(12):1416-e17. doi: 10.1634/theoncologist.2016-0296. Epub 2016 Nov 30. Oncologist. 2016. PMID: 27903924 Free PMC article. Clinical Trial.
-
A systematic review of antibody-drug conjugates and bispecific antibodies in head and neck squamous cell carcinoma and nasopharyngeal carcinoma: Charting the course of future therapies.Cancer Treat Rev. 2024 Jul;128:102772. doi: 10.1016/j.ctrv.2024.102772. Epub 2024 May 26. Cancer Treat Rev. 2024. PMID: 38820656
-
Afatinib in squamous cell carcinoma of the head and neck.Expert Opin Pharmacother. 2016 Jun;17(9):1295-301. doi: 10.1080/14656566.2016.1183647. Epub 2016 May 19. Expert Opin Pharmacother. 2016. PMID: 27160335 Review.
Cited by
-
Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs.Chem Rev. 2016 Dec 14;116(23):14379-14455. doi: 10.1021/acs.chemrev.6b00209. Epub 2016 Nov 23. Chem Rev. 2016. PMID: 27960273 Free PMC article. Review.
-
An Exploratory Study of Refining TNM-8 M1 Categories and Prognostic Subgroups Using Plasma EBV DNA for Previously Untreated De Novo Metastatic Nasopharyngeal Carcinoma.Cancers (Basel). 2022 Apr 11;14(8):1923. doi: 10.3390/cancers14081923. Cancers (Basel). 2022. PMID: 35454830 Free PMC article.
-
Non-metabolic role of UCK2 links EGFR-AKT pathway activation to metastasis enhancement in hepatocellular carcinoma.Oncogenesis. 2020 Dec 4;9(12):103. doi: 10.1038/s41389-020-00287-7. Oncogenesis. 2020. PMID: 33277463 Free PMC article.
-
In Silico Discovery of Potential Uridine-Cytidine Kinase 2 Inhibitors from the Rhizome of Alpinia mutica.Molecules. 2016 Apr 8;21(4):417. doi: 10.3390/molecules21040417. Molecules. 2016. PMID: 27070566 Free PMC article.
-
Metronomic oral cyclosphosphamide as third-line systemic treatment or beyond in patients with inoperable locoregionally advanced recurrent or metastatic nasopharyngeal carcinoma.Medicine (Baltimore). 2017 Apr;96(15):e6518. doi: 10.1097/MD.0000000000006518. Medicine (Baltimore). 2017. PMID: 28403082 Free PMC article. Clinical Trial.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. - PubMed
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. - PubMed
-
- Samlowski WE, Moon J, Kuebler JP, et al. Evaluation of the combination of docetaxel/carboplatin in patients with metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN): a Southwest Oncology Group Phase II study. Cancer Invest. 2007;25:182–188. - PubMed
-
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116–1127. - PubMed
-
- Chan AT, Gregoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl. 7):vii83–vii85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

