Genetically targeted optical electrophysiology in intact neural circuits
- PMID: 23932121
- PMCID: PMC3874294
- DOI: 10.1016/j.cell.2013.07.027
Genetically targeted optical electrophysiology in intact neural circuits
Abstract
Nervous systems process information by integrating the electrical activity of neurons in complex networks. This motivates the long-standing interest in using optical methods to simultaneously monitor the membrane potential of multiple genetically targeted neurons via expression of genetically encoded fluorescent voltage indicators (GEVIs) in intact neural circuits. No currently available GEVIs have demonstrated robust signals in intact brain tissue that enable reliable recording of individual electrical events simultaneously in multiple neurons. Here, we show that the recently developed "ArcLight" GEVI robustly reports both subthreshold events and action potentials in genetically targeted neurons in the intact Drosophila fruit fly brain and reveals electrical signals in neurite branches. In the same way that genetically encoded fluorescent sensors have revolutionized the study of intracellular Ca(2+) signals, ArcLight now enables optical measurement in intact neural circuits of membrane potential, the key cellular parameter that underlies neuronal information processing.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

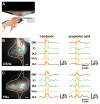

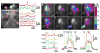

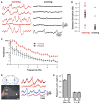
Comment in
-
Genetically encoded voltage sensor goes live.Nat Biotechnol. 2013 Nov;31(11):994-5. doi: 10.1038/nbt.2738. Nat Biotechnol. 2013. PMID: 24213775 No abstract available.
References
-
- Akemann W, Mutoh H, Perron A, Rossier J, Knöpfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods. 2010;7:643–649. - PubMed
-
- Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knöpfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol. 2012;108:2323–2337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

