Sculpting the blank slate: how fibrin's support of vascularization can inspire biomaterial design
- PMID: 23933102
- PMCID: PMC3864148
- DOI: 10.1016/j.actbio.2013.07.043
Sculpting the blank slate: how fibrin's support of vascularization can inspire biomaterial design
Abstract
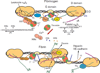
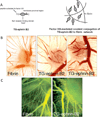
Fibrin is the primary extracellular constituent of blood clots, and plays an important role as a provisional matrix during wound healing and tissue remodeling. Fibrin-based biomaterials have proven their utility as hemostatic therapies, scaffolds for tissue engineering, vehicles for controlled release, and platforms for culturing and studying cells in three dimensions. Nevertheless, fibrin presents a complex milieu of signals to embedded cells, many of which are not well understood. Synthetic extracellular matrices (ECMs) provide a blank slate that can ostensibly be populated with specific bioactive cues, including growth factors, growth factor binding motifs, adhesive peptides and peptide crosslinks susceptible to proteases, thereby enabling a degree of customization for specific applications. However, the continued evolution and improvement of synthetic ECMs requires parallel efforts to deconstruct native ECMs and decipher the cues they provide to constituent cells. The objective of this review is to reintroduce fibrin, a protein with a well-characterized structure and biochemistry, and its ability to support angiogenesis specifically. Although fibrin's structure-function relationships have been studied for decades, opportunities to engineer new and improved synthetic hydrogels can be realized by further exploiting fibrin's inspiring design.
Keywords: Angiogenesis; Biomaterial; Extracellular matrix; Fibrin.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. - PubMed
-
- Clark RA. The molecular and cellular biology of wound repair. Springer; 1996.
-
- Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

